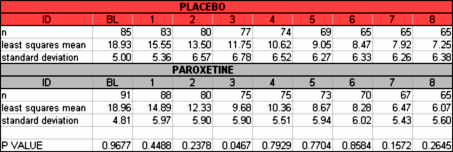
By both parametric and nonparametric analysis, the change in HAM-D total score in the LOCF sample at 8 weeks did not differ between the PXL group and the Placebo group… These analyses were run by me tonight using SigmaStat software…t-test: Sunday, August 26, 2012, 9:41:23 PM
Data source: Data 1 in Notebook1
Normality Test [Shapiro-Wilk] Passed [P = 0.745]
Equal Variance Test: Passed [P = 0.850]
Group Name N Missing Mean Std Dev SEM Placebo dHAM 85 0 9.271 7.129 0.773 PXL dHAM 86 0 10.907 7.589 0.818 Difference -1.636
t = -1.453 with 169 degrees of freedom. [P = 0.148]
95 percent confidence interval for difference of means: -3.860 to 0.587The difference in the mean values of the two groups is not great enough to reject the possibility that the difference is due to random sampling variability. There is not a statistically significant difference between the input groups [P = 0.148].
- the lesson of Study 329: the basics…
- the lesson of Study 329: efficacy drift to trends and 2°s…
- the lesson of Study 329: conventions and protocols…
- the lesson of Study 329: clues and adversities…
- the lesson of Study 329: uh-oh!…
- the lesson of Study 329: data transparency…
- the lesson of Study 329: the authors…
- the lesson of Study 329: the hurdles…
- the lesson of Study 329: naked Emperors, fractious Queens…
ANSWER: I believe I created it on my own.
QUESTION: Okay. Did Martin Keller tell you what to put in the first draft?
ANSWER: I don’t recall. I don t think I had any conversation with him until we were, you know, afterwards.
QUESTION: Okay. After you prepared the first manuscript?
ANSWER: To the best of my recollection, yes.
The lesson I take from Study 329 is in the top half of this post – the importance of public availability of the raw data. It doesn’t take a rocket scientist to read Study 329 and know that there’s something wrong. Just take the paper’s abstract and jot in the p values from Table 2 as I did when I was reviewing it:
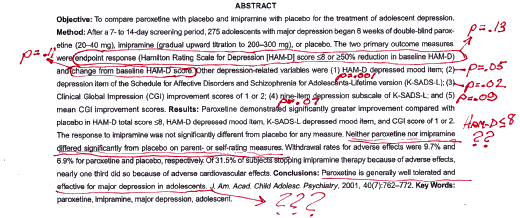
Both primary variables were insignificant, only three of five secondary variables achieved significance, and Table 2 leads with HAM-D < 8, an after-the-fact add-on. What Jureidini and Tonkin got for making this same observation were ad hominem attacks from the authors and the editor. If they had access to the raw data, they could’ve [and would’ve] properly vetted the article and easily exposed the analysis presented by Ms. Laden. In fact, if the data were publicly available, I doubt either GSK, Sally Laden, or the authors would’ve been foolish enough to submit it for publication.
I don’t know how it happened that the raw data from clinical trials came to be considered private property. Articles themselves are published – meaning placed into the public domain. An article is just a wrapper on the data. So why hide the data? As I said earlier, the only reason I can think of is to allow it to be distorted and massaged. So I guess my next blogging forray will be to find out how that got started. I can think up all kinds of reasons that industry might oppose such a change, but none that have to do with patient care which is all that matters. If a clinical trial can’t survive full data disclosure, that tells us all we really need to know. Study 329 is what can happen when primary data is kept secret. It wasn’t at all pretty. And our literature is filled with Study 329s.
If you’ve been reading this series along, you’ll be aware that we’ve heard a lot from an anonymous commenter, someone who is knowledgeable about this case, its issues, and has some suggestions about how to proceed. Rather than trying to summarize his/her position, I’d prefer to change sides and ask him to give us his/her suggestions as a blog post. The arguments are strong and deserve a front page hearing. I’ll add my two cents in the comments to complete the role reversal. I hope he/she will take me up on that offer. But there’s one place where I think we’ll disagree, and that’s in calling for the American Academy of Child and Adolescent Psychiatrists to retract this article. So I’ll end this post with why I continue to feel that the call for retraction stands, at least from me.
I am aware of no comment from any listed author [Martin B. Keller, Neal D. Ryan, Michael Strober, Rachel G. Klein, Stan P. Kutcher, Boris Birmaher, Owen R. Hagino, Harold Koplewicz, Gabrielle A. Carlson, Gregory N. Clarke, Graham J. Emslie, David Feinberg, Barbara Geller, Vivek Kusumakar, George Papatheodorou, William H. Sack, Michael Sweeney, Karen Dineen Wagner, Elizabeth B. Weller, Nancy C. Winters, Rosemary Oakes, and James P. McCafferty] that has publicly acknowledged that this was a ghost-written, industry-directed, jury-rigged article. Dr. Keller stepped down as chairman at Brown in 2009 with no comment and retired two months ago, again with no comment. Mina Dulcan has retained her defensive stance in every public comment, as has her successor as editor, Andres Martin. If any officer of the American Academy of Child and Adolescent Psychiatrists has stated that this article was corrupted or should not have been published, I’ve missed it. Appeals have gone out to the Universities of the listed authors in the article [eg Brown University President Ruth Simmons], and they have been dismissed without comment, most recently by Brown’s new president, child advocate Christine H. Paxson. Yet this article was the centerfold for the recent DOJ case against GSK settled for $3 B, the largest such fine ever levied. And GSK agreed to the allegations as part of the settlement under threat of even harsher action.
Something terrible happened in psychiatry, an alliance between the pharmaceutical industry and a number of our leaders who allowed their academic credentials to be used for commercial purposes. It happened on a large scale, and in the process shamed all of us, whether we were involved or not. Many of the people we looked to to guide us fell into the role of key opinion leaders – a marketing term that meant that they could influence what we did and how we practiced. And in that new role, medical degrees, academic positions, the methods of science, and psychiatric experience became little more than marketing tools in a commercial campaign. Such was the case with Study 329 – an article that has become exemplary because it is now so painfully well known – a symbol for the failings of an era. It needs to be retracted simply because it was wrong on purpose – and we all know it. And yet no involved person, institution, or organization highlighted in that last paragraph has swallowed their pride, or guilt, or denial, or arrogance and called for retraction.
How many weeks are you going to rail about this topic? It was a crap study, the US government is nailing them for some money, and people who have been paying attention know what garbage it was and how academia doesn’t give a crap about anything else but their own special interests and just making money to continue to have influence in a deteriorating society that is just about “gimme the freakin’ drugs so I can think I’m better”.
Can’t medicate life, let the losers who sell the false message of otherwise, let the losers who believe the message, and the losers who do nothing to disspell the message end up where they belong.
Sorry, we really have to hope the entrenched minions of this failed policy of biochemical imbalance just die off, because you cannot reason with them nor find alternative ways to make them irrelevant. I am not advocating people hurt these clueless advocates of “drugs are us”, just that nature takes its course. Because let’s be honest, most of the group behind psychopharmacology gone berserk are just old guys who couldn’t handle that psychiatry was being marginalized and being made irrelevant by both non physicians and colleagues without appropriate training to prescribe, so they falsely created a hollow niche that had no honest basis to be maintained. And doctors with no gonads nor concern for the better welfare of the public they allegedly served just said, “ok”.
And people will think my comment is rude and inappropriate. How ironic, so has been the hierarchy of this profession for the past 15 years or so!
Per Ramses of the Ten Commandments: so let it be written, so let it be done!
How many weeks are you going to rail about this topic?
I guess until it’s retracted….
Rail on, Dr. Mickey. At the very least Study 329 could be an object lesson.
(Perhaps those spiteful and defensive behaviors attributed to children by adults are adult behaviors after all.)
Good luck waiting for that to happen. Maybe about the same time PPACA will be repealed. Again, no change in who really runs the show in our profession will not lead to efficacious care. Whores and cowards, there’s the name for a blog about psychiatry.
These tampered results of single drugs is just the beginning. Having been convinced at one point that I would eventually be psychotic for life if I didn’t stay on psychiatric drugs that caused more problems than I ever had before, I’ve learned that when combined with other drugs, a drug can behave very differently. With rotating polypharmacy NOBODY KNOWS what is being done to the lives and brains of people who have been labeled as mentally ill. What is the personal problem, the social problem, the cognitive problem, the brain problem, or the drug problem is impossible to sort and piling on more drugs just makes it worse. The idea that none of those matter, but the brain problem and that all should be made well with the right algorithm is insulting to the complexities of being a member of the human race.
It seems perfectly possible to me that there can be change in the mental health field. The recovery movements in the U.K. are something that could work in the U.S. well.
So, rock on Mickey. I read all your posts and enjoy them.
Transparency for Clinical Trials — The TEST Act
Jeffrey M. Drazen, M.D.
N Engl J Med 2012; 367:863-864August 30, 2012
“This legislation is important. The bill requires that any trial that could be used to support an application for FDA approval be registered in ClinicalTrials.gov and that the results be reported in a timely fashion. It requires that early-phase trials (those in which a drug is initially tested in humans) be registered. Thus, these trials will become public knowledge.”
“We can make progress in medicine only if people are willing to put themselves at risk to test new diagnostic and therapeutic approaches. To recognize and reward these participants, and in keeping with the Declaration of Helsinki, clinical trials should be conducted in the open, with full public knowledge of the question asked, the intervention tested, and the results obtained. The TEST Act is another step toward this end, and we strongly support it.