A Randomized, Double-Blind Placebo-Controlled Trial of Oral Creatine Monohydrate Augmentation for Enhanced Response to a Selective Serotonin Reuptake Inhibitor in Women With Major Depressive Disorder
by In Kyoon Lyoo, Sujung Yoon, Tae-Suk Kim, Jaeuk Hwang, Jieun E. Kim, Wangyoun Won, Sujin Bae, and Perry F. Renshaw
American Journal of Psychiatry. 2012 169:937–945.
Objective: Antidepressants targeting monoaminergic neurotransmitter systems, despite their immediate effects at the synaptic level, usually require several weeks of administration to achieve clinical efficacy. The authors propose a strategy of adding creatine monohydrate [creatine]) to a selective serotonin reuptake inhibitor [SSRI] in the treatment of patients with major depressive disorder. Such augmentation may lead to a more rapid onset of antidepressant effects and a greater treatment response, potentially by restoring brain bioenergetics at the cellular level.
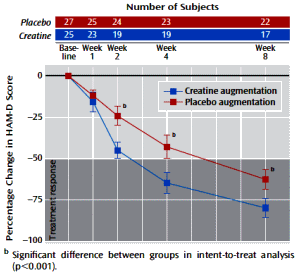
Method: Fifty-two women with major depressive disorder were enrolled in an 8-week double-blind placebo-controlled clinical trial and randomly assigned to receive escitalopram in addition to either creatine [5 g/day, N=25] or placebo [N=27]. Efficacy was primarily assessed by changes in the Hamilton Depression Rating Scale [HAM-D] score.
Results: In comparison to the placebo augmentation group, patients receiving creatine augmentation showed significantly greater improvements in HAM-D score, as early as week 2 of treatment. This differential improvement favoring creatine was maintained at weeks 4 and 8. There were no differences between treatment groups in the proportion of patients who discontinued treatment prematurely [creatine: N=8, 32.0%; placebo: N=5, 18.5%] or in the overall frequency of all reported adverse events [creatine: 36 events; placebo: 45 events].
Conclusions: The current study suggests that creatine augmentation of SSRI treatment may be a promising therapeutic approach that exhibits more rapid and efficacious responses in women with major depressive disorder.


But my main point is about that Tab that says No Study Results Posted. We wouldn’t expect it to say anything else under the current system with this particular study. Results are only required for certain kinds of studies, and they’re only required to be posted within a year of the end. Here’s what the header for the Results page looks like when the results are in:

It’s the study with the locations above [An Open-Label Comparison of Duloxetine to Other Alternatives for the Management of Diabetic Peripheral Neuropathic Pain]. If you scroll down that page, it has the study results. There’s a lot there – a whole lot! It’s pretty impressive and I can see why they give them a year to post their results. It looks like a lot of work to put together.
Sorry, the comment form is closed at this time.