A Double-blind, Randomized, Placebo-Controlled Trial of Fluoxetine in Children and Adolescents With Depression
by Graham J. Emslie, MD; A. John Rush, MD; Warren A. Weinberg, MD; Robert A. Kowatch, MD; Carroll W. Hughes, PhD; Tom Carmody, PhD; and Jeanne Rintelmann
Archives of General Psychiatry. 1997, 54:1031-1037.
Background: Depression is a major cause of morbidity and mortality in children and adolescents. To date, randomized, controlled, double-blind trials of antidepressants (largely tricyclic agents) have yet to reveal that any antidepressant is more effective than placebo. This article is of a randomized, double-blind, placebo controlled trial of fluoxetine in children and adolescents with depression.
Method: Ninety-six child and adolescent outpatients (aged 7-17 years) with nonpsychotic major depressive disorder were randomized (stratified for age and sex) to 20 mg of fluoxetine or placebo and seen weekly for 8 consecutive weeks. Randomization was preceded by 3 evaluation visits that included structured diagnostic interviews during 2 weeks, followed 1 week later by a 1-week, single-blind placebo run-in. Primary outcome measurements were the global improvement of the Clinical Global Impressions scale and the Children’s Depression Rating Scale – Revised, a measure of the severity depressive symptoms.
Results: Of the 96 patients, 48 were randomized to fluoxetine treatment and 48 to placebo. Using the intent to treat sample, 27 (56%) of those receiving fluoxetine and 16 (33%) receiving placebo were rated "much" or "very much" improved on the Clinical Global Impressions scale at study exit (chi 2=5.1, df=1, P=.02). Significant differences were also noted in weekly ratings of the Children’s Depression Rating Scale – Revised after 5 weeks of treatment (using last observation carried forward). Equivalent response rates were found for patients aged 12 years and younger (n=48) and those aged 13 years and older (n=48). However, complete symptom remission (Children’s Depression Rating Scale – Revised) occurred in only 31% of the fluoxetine-treated patients and 23% of the placebo patients.
Conclusion: Fluoxetine was superior to placebo in the acute phase treatment of major depressive disorder in child and adolescent outpatients with severe, persistent depression. Complete remission of symptoms was rare.
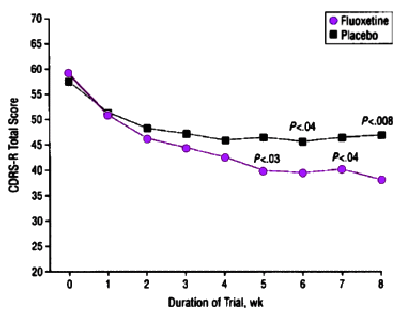
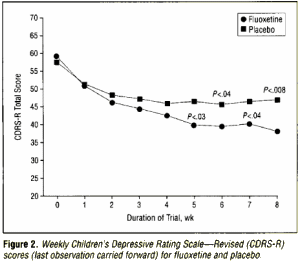
The other primary outcome measure, the weekly CDRS-R score, was examined as a continuous variable. Figure 2 shows the traditional method of dealing with patient attrition during the course of the treatment [ie, weekly CDRS-R scores for each group are presented with the LOCF]. The last available observation is filled in for the values for patients who discontinued study participation before 8 weeks… Comparing the weekly CDRS-R scores for each treatment group using t tests, the first week that the groups were significantly different was week 5. At week 5, the mean CDRS-R score for the fluoxetine-treated group [39.8±13.2] was lower than the placebo group [46.8±16.6] [t=-2.28, df=94, P=.03]. To make the most efficient use of the available data without resorting to a completion analysis or LOCF analysis, the rate of change [slope] and baseline CDRS-R score [intercept]) were estimated from linear regressions on each patient and for each group. The estimated baselines were similar [54.2 for the fluoxetine-treated group vs 53.8 for the placebo group]. However, the fluoxetine-treated group slope of -2.75±-2.52 was significantly different from the placebo group slope of -1.27±-2.86 [t=2.68, df=94, P<.001].These results may be interpreted as follows. The fluoxetine-treated group began with an average CDRS-R score of 54.2 and their scores improved by 2.75 U per week to end with an estimated week 8 score of 32.2. While placebo patients began with a similar average CDRS-R score [53.8], their score improved only 1.27 U per week to the end of the study with an estimated exit score of 43.6. Using the empirical Bayesian analysis of estimating slopes [intercept estimates were unavailable] gives an estimated slope of -2.60±2.36 for the fluoxetine treated group and a significantly smaller slope of -1.32±3.56 for the placebo group [t=2.08, df=94,P=.04].
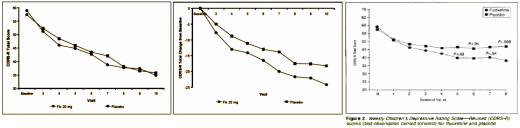
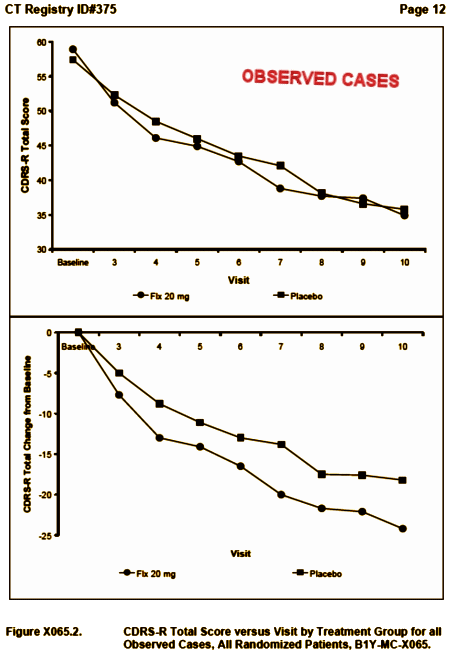
Study X065 was the first Clinical Trial of an SSRI [Prozac] in Adolescents. It was one of the two studies used by Lilly to get approval for Prozac in the treatment of MDD in Adolescents [the only SSRI approved for kids since it got through the FDA before the black box warning was imposed]. To my knowledge, the only sight we have of the primary raw data from this study is the top graph in Figure X065.2 above. The published graph [LOCF] and the % change lower graph are ways to make things look better on paper. And the verbiage in the article [quoted above] is an indirect way of claiming a difference using regression slopes, in lieu of showing us that simple raw data graph [which clearly shows no difference].
So that’s why I want the raw data. If I had seen that top X065.2 graph, I would’ve simply said, Prozac doesn’t help depressed kids, no matter what the manipulated versions showed. So would you. That’s why they didn’t show it. There’s a second point. Gibbons et al used this data in his meta-analysis that said the SSRIs were efficacious in adolescents. All I see is even more evidence that his meta-analysis is flawed…
[what they saw] [what we saw]
One of the things that has resulted from Carper’s investigation is the AACAP has been developing things like, “A Guide for Community Child Serving Agencies on Psychotropic Medications for Children and Adolescents” it states, “Some psychotropic medications have FDA Black Box Warnings. Medicines with black box warnings are still FDA approved, but their use requires particular attention and caution regarding potentially dangerous or life threatening side effects. Selective Serotonin Reuptake Inhibitors (SSRI’s) carry a black box warning that they may cause suicidal ideation or behavior, although the most recent review of the evidence is not conclusive that SSRIs increase suicidal behavior.” The only one I know of is Gibbon’s. http://ow.ly/dGnAS
Thanks Becky,
PRESS RELEASE aacap:
aacap A Guide for Community Child Serving Agencies on Psychotropic Medications for Children and Adolescents
NOTE: This Guide is dated February 2012
Funny how the Observed Cases (completers) don’t look like the LOCF sample. That’s counterintuitive at best and it calls into question the validity of the study.