This is a follow-up to the UPDATE: at the end of shoveling…:
J&J’s Alex Gorsky testify? Much too busy … on the other side of the planet
Philadelphia Inquirer
By David Sell
September 19, 2012
People will just have to understand that Johnson & Johnson chief executive officer Alex Gorsky has places to go. He does not have time to testify at trials, to talk about the past and allegations of inappropriate marketing of Johnson & Johnson’s antipsychotic drug Risperdal. But what about Exelon, the Novartis Alzheimer’s drug? Wasn’t that Gorsky’s name atop a warning letter from the U.S. Food and Drug Administration in 2007, when Gorsky led Novartis’ U.S. pharmaceutical division. Indeed it was, as the FDA asked Gorsky to explain why one of the company’s promotional materials, "makes unsubstantiated superiority claims for Exelon, overstates the efficacy of Exelon, includes misleading risk presentations, and recommends or suggests a combination use of Exelon that has not been approved by FDA." A link to that 2007 letter is here.The funny thing is that the warning letter to Gorsky at Novartis in 2007 sounded very similar to the one sent to Janssen Pharmaceutica CEO Ajit Shetty on April 19, 2004. Janssen is the J&J subsidiary that makes Risperdal and Shetty reported to Gorsky, who departed J&J in the fall of 2005 to guide Novartis’ US pharmaceutical business. The 2004 warning letter on Risperdal said there was a serious problem because a Janssen letter to health-care providers "misleadingly omits material information about Risperdal, minimizes potentially fatal risks associated with the drug, and claims superior safety to other drugs in its class without adequate substantiation." A link to the 2004 letter is here.
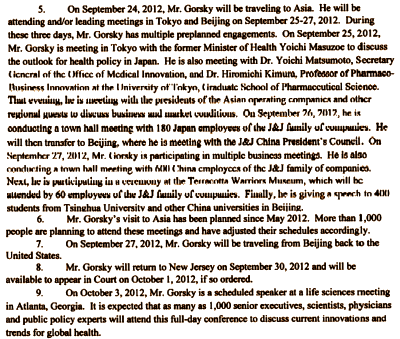
The next in a string of the trials is scheduled to start Monday Philadelphia Court of Common Pleas. The lawsuit was filed by the parent of a 17-year-old boy from Sherman, Tex., who was prescribed and given Risperdal when he was only five years old. He was 12 years old when he started developing breasts. Just before the first trial began, J&J settled rather than risk having a judge rule that Gorsky had to testify. Plaintiffs attorneys Stephen Sheller and Brian McCormick had deposed Gorsky in May, so J&J’s attorneys at Drinker, Biddle knew at least some of what would be discussed in front of the jury that had been picked. This time, they tried another way. Late on Friday, they played the travel card. They filed a deposition from Gorsky’s assistant, Kathleen Torok, who very clearly explained that Gorsky would not be available to testify on Sept. 26 because he is scheduled to be in Japan and China and that’s all there is to it. Lest the note be read as a complete brush-off to the judge, Torok mentions in the eighth bullet point, "Mr. Gorsky will return to New Jersey on September 30 and will be available to appear in Court on Oct. 1, 2012, if so ordered." Torok’s deposition and Gorsky’s travel plans are here.
Sheller and McCormick were not thrilled at word of Gorsky’s plans to visit with J&J folks in Asia. They were very suspicious of Torok’s suggestion the plans were in place since May and they hadn’t heard about this issue before. They asked the judge to order Gorsky to show up in court on Friday to explain himself. Their reply to the J&J motion that Gorsky’s subpoena should be quashed is here.
See, I told you the sarcasm could be infectious. At least I didn’t quip that the Terra Cotta Warriors are used to waiting. They’ve waited for centuries.
The reason for all the sarcasm is that we’re increasingly aware that this whole Risperdal story is a fairly low-brow affair. J&J bought their way into the public market for Schizophrenia with the TMAP scheme. They tried to get into the pediatric market with behavior control, and when that didn’t fly, they jumped on Biederman’s Bipolar Child mania, even getting him to sign on to a rewrite of their earlier behavior study. They marketed the drugs for kids without FDA approval. They used the notorious Dr. Nemeroff for an augmentation in adult treatment resistant depression study that was quickly debunked. They even sold it to the elderly after being waved off by the FDA, in spite of the fact that it turned out to be ineffective and toxic. And all along the way, they minimized adverse effects: elevated Prolactin levels, obesity, diabetes. At every level, their claims were trumped up and deceptive. Alex Gorsky was with Risperdal every step of the way. He took Risperdal to the top of the charts, and Risperdal did the same for him.

Sorry, the comment form is closed at this time.