When Clinical Trials are Meant for Marketing, not Science
Healthcare Renewal
by Roy Poses
October 19, 2012
The development of the randomized controlled clinical trial (RCT) was one of the major scientific advances in clinical medicine. RCTs provide a major part of the evidence underlying evidence based medicine. RCTs provide a major source of data used by the US Food and Drug Administration, and similar agencies in other countries, to decide whether to approve drugs or devices to manage particular clinical problems. Unfortunately, with the rise of the RCT came a rise in attempts to suppress and manipulate clinical trials by those with vested interests, often in selling the products and services the trials can evaluate.Many of the examples we have discussed were of attempts at manipulation or suppression of clinical trials originally done to provide evidence for product approval, or even ostensibly to advance clinical science. Yet a relatively new article covering evidence revealed from litigation about the drug gabapentin (Neurontin) originally made by Parke-Davis, first a part of Warner Lambert, now part of Pfizer suggests that many clinical trials may not be done to advance science, or even just to provide data to regulators, but only to market products…
Implementation of a publication strategy in the context of reporting biases. A case study based on new documents from Neurontin® litigation
by S S Vedula, Palko S Goldman, Ilyas J Rona, Thomas M Greene and Kay Dickersin1
Trials. 2012, 13:136.
[full text online]
Background: Previous studies have documented strategies to promote off-label use of drugs using journal publications and other means. Few studies have presented internal company communications that discussed financial reasons for manipulating the scholarly record related to off-label indications. The objective of this study was to build on previous studies to illustrate implementation of a publication strategy by the drug manufacturer for four off-label uses of gabapentin (Neurontin®, Pfizer, Inc.): migraine prophylaxis, treatment of bipolar disorders, neuropathic pain, and nociceptive pain.Methods: We included in this study internal company documents, email correspondence, memoranda, study protocols and reports that were made publicly available in 2008 as part of litigation brought by consumers and health insurers against Pfizer for fraudulent sales practices in its marketing of gabapentin (see webcite for the Court’s findings).
We reviewed documents pertaining to 20 clinical trials, 12 of which were published. We categorized our observations related to reporting biases and linked them with topics covered in internal documents, that is, deciding what should and should not be published and how to spin the study findings (re-framing study results to explain away unfavorable findings or to emphasize favorable findings); and where and when findings should be published and by whom.Results: We present extracts from internal company marketing assessments recommending that Pfizer and Parke-Davis (Pfizer acquired Parke-Davis in 2000) adopt a publication strategy to conduct trials and disseminate trial findings for unapproved uses rather than an indication strategy to obtain regulatory approval. We show internal company email correspondence and documents revealing how publication content was influenced and spin was applied; how the company selected where trial findings would be presented or published; how publication of study results was delayed; and the role of ghost authorship.
Conclusions: Taken together, the extracts we present from internal company documents illustrate implementation of a strategy at odds with unbiased study conduct and dissemination. Our findings suggest that Pfizer and Parke-Davis’s publication strategy had the potential to distort the scientific literature, and thus misinform healthcare decision-makers.
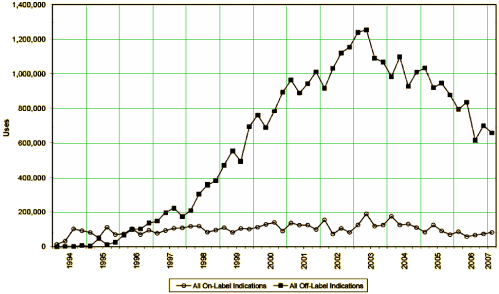
I guess this graph is the bottom line – the sales of Neurontin on-label versus off-label in response to the campaign outlined in these articles. Dr. Poses piece does a fine job of laying out how Parke-Davis and Pfizer systematically set up a program for publishing articles as infomercials in our peer reviewed literature to achieve such lucrative results [without even risking getting turned down by the FDA].
I know we’re getting used to reading this stuff: Janssen’s Excerpta Medica around the corner with more ghostwritten articles than recruited KOLs to sign on; Sally Laden and GSK’s James McCafferty passing the Paxil Study 329 back and forth for review; STI’s Sally Laden handing off Nemeroff’s and Schatzberg’s textbook to Diane Coniglio because she was so busy to ghostwrite it herself; Parke-Davis’ Marketing team proofreading ghostwritten Neurontin articles for grammar and accurate branding message to move them along quickly. They treated our scientific medical literature like it was their playground for making the prescribing habits of physicians conform to their marketing plans. It was widespread and went on for years. From the released documents in this article, it doesn’t sound like they even thought about what they were doing – they spoke with each other like they had a perfect right to be jury-rigging our literature, a right to twist and turn the facts for their branding needs, just business as usual. Somehow, this case feels even worse than the others: the matter-of-fact way they approach what they’re doing; the business plan detached from medical care or the regulatory system that governs us. It’s just a marketing success story – how to turn a middle weight anticonvulsant into a blockbuster psychiatric drug. Who needs the FDA when you’ve got a cracker-jack marketing team like this? And notice that they simply assumed they could get what they needed published wherever they needed it to be to stay on schedule.
As the extent of the corruption introduced into the psychiatric literature by the pharmaceutical companies and the key role played by academic psychiatrists becomes increasingly evident, the silence from the Universities, the Medical Schools, the Journals, and our professional organizations becomes louder and louder. This Neurontin story is not an exception, any more than the Risperdal story, or the Zyprexa story, or the Paxil story, etc. are exceptions. Some version of this narrative has been the rule for several decades. We hear over and over that this problem won’t be resolved until stiffer penalties are levied on industry, until the actual executives directing this kind of behavior are punished, until we have more regulation and oversight in place. I don’t disagree with any of those measures, but they locate the responsibility for integrity and medical ethics outside the domain of organized and academic medicine. That’s an error.
Thank you so much—I really need to see/hear “But what we really need are people at the top who are doctors. Real doctors wouldn’t put up with this shit, much less be a part of it…” It isn’t enough to know this to be true—I’ve been awake and living a nightmare in real life knowing that pretty much everyone is asleep—hearing it from you doesn’t make the nightmare go away, but knowing you see what I see somehow makes it not as bad…
fyi
http://psychnews.psychiatryonline.org/newsArticle.aspx?articleid=1384224
Pediatric Prescriptions for SGAs Most Commonly for Off-Label Uses
Mark Moran
Nearly two-thirds of children on Medicaid receiving prescriptions for second-generation antipsychotics did so for disorders for which regulatory approval has not been granted.
Dr. Nardo
Thank you again for your work. I have thought for many years that psychiatry needs to accept responsibility for its role in this debacle of modern medicine.
“Real doctors wouldn’t put up with this shit, much less be a part of it…”
If only it were so. While they may not be actively engaged, far too many doctors remain silent in the face of obvious corruption. They know if they speak out they will invite the wrath of their “unions” (the APA, AMA, AAP, etc.) and state medical boards. (That’s a nice practice you have there, it would be a shame if anything happened to it…) It’s much easier to just keep a low profile, not exactly profiles in courage.
Why are psychiatrists suckers for drug fads?
Note: Although it may be useless in treating a particular individual, Neurontin can also be difficult to discontinue and requires tapering.