Anyone familiar with GlaxoSmithKline and Paxil will know all about the Paxil 329 study that they [Glaxo] hired a PR firm to draft and, later, persuaded key thought leaders, mainly child psychiatrists to endorse and promote at any given opportunity. The 329 study has been dissected by many who are in agreement that it’s a piece of fraud. Read more about the Paxil 329 study here and here. Not content with one study doing the rounds in various journals and pediatric clinics Glaxo, in their infinite wisdom, hired the services, once again, of a ghostwriting team to pimp out the paroxetine 352 bipolar trial."Study 352" was published in the American Journal of Psychiatry (158:906-912; June 2001) and, like all good ghostwritten articles, suggested that Paxil may be beneficial in the treatment of bipolar depression. The study cited Charles B. Nemeroff as the lead author. The name Nemeroff is synonymous in the ghostwriting and kickback field. He’s made a lucrative living out of speaker fees and pharmaceutical roundtables, although this is classed as ‘honoraria’ [ex gratia payment].
Okay, we are all entitled to make a bit on the side, all entitled to be paid for our expertise. When that expertise, however, is constructed, conceived, call it what you will, by somebody else then that honoraria should either be given back, paid to the owner of the said work or given to a charity where the said work/opinion has harmed a person/s. For that one would need a conscience. My opinion, for what it’s worth, neither Glaxo or Nemeroff have a conscience.
Ironically, Glaxo hired the same PR outfit, Scientific Therapeutics Information, [STI] to misrepresent information from Study 352. STI also drafted the original Paxil 329 study, specifically Sally Laden. Just like Study 329, 352 also made unsubstantiated efficacy claims and downplayed the adverse event profile of Paxil…
Double-blind, placebo-controlled comparison of imipramine and paroxetine in the treatment of bipolar depression.
by Nemeroff CB, Evans DL, Gyulai L, Sachs GS, Bowden CL, Gergel IP, Oakes R, and Pitts CD.
American Journal of Psychiatry. 2001 158[6]:906-912.
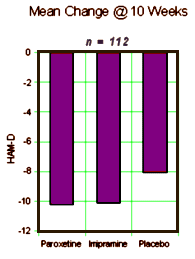
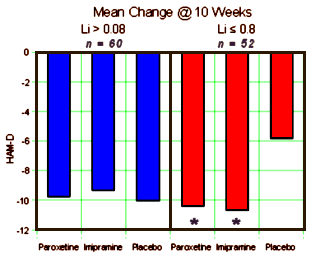
OBJECTIVE: This study compared the efficacy and safety of paroxetine and imipramine with that of placebo in the treatment of bipolar depression in adult outpatients stabilized on a regimen of lithium.METHOD: In a double-blind, placebo-controlled study, 117 outpatients with DSM-III-R bipolar disorder, depressive phase, were randomly assigned to treatment with paroxetine (N=35), imipramine (N=39), or placebo (N=43) for 10 weeks. In addition to lithium monotherapy, patients may have received either carbamazepine or valproate in combination with lithium for control of manic symptoms. Patients were stratified on the basis of trough serum lithium levels determined at the screening visit (high: >0.8 meq/liter; low: </=0.8 meq/liter). Primary efficacy was assessed by change from baseline in scores on the Hamilton Rating Scale for Depression and the Clinical Global Impression illness severity scale.RESULTS: Differences in overall efficacy among the three groups were not statistically significant. For patients with high serum lithium levels, antidepressant response at endpoint also did not significantly differ from placebo. However, both paroxetine and imipramine were superior to placebo for patients with low serum lithium levels. Compared to imipramine, paroxetine resulted in a lower incidence of adverse events, most notably emergence of manic symptoms.CONCLUSIONS: Antidepressants may not be useful adjunctive therapy for bipolar depressed patients with high serum lithium levels. However, antidepressant therapy may be beneficial for patients who cannot tolerate high serum lithium levels or who have symptoms that are refractory to the antidepressant effects of lithium.
The paroxetine 352 bipolar trial: A study in medical ghostwriting
by Jay D. Amsterdam and Leemon B. McHenry
International Journal of Risk & Safety in Medicine 2012 24:221–231.
Background: The problem of ghostwriting in corporate-sponsored clinical trials is of concern to medicine, bioethics, and government agencies. We present a study of the ghostwritten archival report of an industry-sponsored trial comparing antidepressant treatments for bipolar depression: GlaxoSmithKline (GSK) paroxetine study 352. This analysis is based upon publicly available evidence presented in a complaint of research misconduct filed with the Office of Research Integrity of the Department of Health and Human Services.Objectives: We performed a deconstruction of the published study to show how primary and secondary outcome analyses were conflated, turning a ‘negative’ clinical trial into a ‘positive’ study – with conclusions and recommendations that could adversely affect patient health.Methods: The paroxetine 352 study was a randomized, double-blind, placebo-controlled, 19-site trial comparing paroxetine and imipramine in 117 patients with bipolar type I major depressive episode which was unresponsive to prior lithium carbonate therapy.Results: Analysis of the primary outcome measures found no statistically significant difference between paroxetine or imipramine versus placebo. However, the published article concluded that both drugs were efficacious versus placebo for a post hoc subgroup of patients.Conclusions: Few industry-sponsored studies gain public scrutiny. It is important to make these articles transparent to the scientific and medical community.
And Paxil [Seroxat] itself isn’t benign. If my experience is any indicator, besides the other problems of SSRIs, it has the most malicious and prolonged withdrawal syndrome of the lot. When my childhood chum calls about his daughter who was put on Paxil at 14 for some adolescent blues and is still on it at 23, anorgasmic, unable to tolerate stopping it with multiple attempts, calling from an Emergency room where she was suicidally depressed from withdrawal symptoms – what am I to say to him? "At least she didn’t become suicidal when she started it"? And I know the original prescriber, a decent sort who genuinely wants to help his patients. And I know the guy who tried to detox her this time [in two weeks!], a former colleague. Both were affiliated with Charlie Nemeroff’s former Department, as am I. In my case, Scandal Fatigue is a defense against rage – and often it doesn’t work. The night my friend called, was one of those times.
Seroxat/paxil is pernicious, it is an insidious and subtle poison. When withdrawn abruptedly, in two weeks (!) or less, it creeps into every fibre of your life blood. It chips away and daily, thereafter, a new sympton appears. So, day one you might get head zaps, day two you might get nightmares, day three you might get delirium, day four you might get fear of loud noises and lights, day five you might get anorexia, day six you might get fatigue, day seven you might get agitation, day eight you might get panic, day nine you might get tinnitus, day ten you might get confusion, day ten you might get detachment, day eleven you might get tremors, day eleven you might get aggressive, day twelve you might get akathisia, day thirteen you might get angry, day fourteen you might get aggressive, day fifteen you might attack someone, day sixteen you might kill someone, day seventeen you might not be here anymore.
This is why gps and psychiatrists presented with this list of endless, excruciating, symptoms from seroxat withdrawal cannot cope with it, so, to them, the only course available is benzos and beta-blockers. They are presented with a patient with so many weird and unimaginable symptoms, the list I gave is by no means exhaustive, that it sort of ‘blows their mind’ and one is sent away with yet another prescription which has yet another ‘list’ of ‘mind-blowing’ side-effects.
It is like being a ‘freak of nature’, it is like being in an alien world, and nothing, but nothing, will ever get us back to where we where. It is just too horrific.
Gracias Mikey. Hope you had a good fill of turkey.
I hear Nemeroff’s turkey laid a golden egg.
Re your friend’d daughter. Flick me an email
Boring old woman:
///nothing, will ever get us back to where we where///
Time and peer or/and professionnal support might do-although tapering off should be a message send.
Will Hall, an activist I really
Boring old woman:
///nothing, will ever get us back to where we where///
Time and peer or/and professionnal support might do-although tapering off should be a message send.
Will Hall, an activist I really admire and learn much from-its writing and website that is- gave recently some readers a fear when he advocated going cold turkey in some situation:
Gradual Reduction is Best For Coming Off Meds: But In All Situations?
http://www.madinamerica.com/2012/11/medication-withdrawal-or-medicationtapering-a-harm-reduction-approach/
Dr. Mickey, about your friend’s daughter: Since she’s had multiple unsuccessful withdrawal attempts, her nervous system might be hypersensitive to dosage changes.
Paxil comes in a liquid form. It can be titrated by .01mg if need be.
Suggest an initial trial reduction of 5% per month. If that is tolerated, try 10% per month, calculated on the last dosage. (The absolute amount of the decrements gets progressively smaller.)
When the person decreases to low doses, usually less than 10mg, drops in dosage often get more difficult. At that point, a microtaper of .01mg every 4 days may be possible.
It’s important in the withdrawal attempt not to tip the nervous system into dysregulation, a withdrawal syndrome that can last a very long while, thus the very gradual and tentative reductions. There is no guarantee this woman will regain her sexual functioning, or even be able to get completely off Paxil.
Under no circumstances should someone who is otherwise stable on psychiatric medications (even if he or she has been living with adverse effect for years) go cold turkey.
Alto,
You know I am on your side on tapering off-as is Boring OM, by the way according to a post long enough ago where he explained how he tried to taper off ludicrous prescription lists starting by the least useful if my memory is correct.
Still, on some medical emergencies your doctor has to go cold turkey for you- neuroleptic malignant syndrome and other conditions….
Please Alto.
PS: With respect for your good work, I do not believe that boring and I are the only two worldwide psychiatrists who prescribe tapering off as you wrote on MIA.
Alto,
As you wrote in the sense that you wrote
“only one or two (psychiatrists) in the world even attempt to treat it.” that is;
Sorry about that imprecision.
All the best for your good work.