A Meta-analysis of Factors Impacting Detection of Antidepressant Efficacy in Clinical Trials: The Importance of Academic Sites
by Dunlop BW, Thase ME, Wun CC, Fayyad R, Guico-Pabia CJ, Musgnung J, and Ninan PT.
Neuropsychopharmacology. 2012 37[13]:2830-2836.
Variability in placebo response greatly complicates the design, conduct, and interpretation of clinical trials of antidepressant medications. To identify factors that impact detection of antidepressant-placebo differences, we conducted a meta-analysis of all relevant phase II-IV clinical trials for major depressive disorder conducted by the manufacturer of venlafaxine and desvenlafaxine completed by March 2011. We examined 15 factors potentially relevant to trial outcomes, using the standardized mean difference on the Hamilton Rating Scale for Depression [HAM-D17] score as the primary outcome. Thirty trials comprising 8933 patients were included. In univariate analyses, antidepressant efficacy [ie, drug vs placebo difference] was predicted most strongly [β=3.74, p=0.0002] by the proportion of patients in the trial enrolled from academic sites. Other factors predicting larger drug-placebo differences included lower participant completion rate, fewer post-baseline study visits, earlier year of study, and study drug [venlafaxine>desvenlafaxine]. In multivariate meta-regression modeling, only the proportion of patients from academic sites maintained statistical significance as a predictor of drug-placebo separation for both HAM-D17 continuous score change [β=2.24, p=0.034] and response rate [β=2.26, p=0.035]. Including a higher proportion of academic sites may increase the ability to detect differences between active drug and placebo in clinical trials of major depressive disorder.
Not my usual fare – an industry-funded, industry-friendly/industry-employee authored, industry-data-based study – but it’s by a friend, and it makes a point that is related to my last post, a point that should’ve been obvious thirty years ago when the CRO industry was getting its start. The CRO Trials using private Clinical Research Centers [CRCs] don’t do the job very well. I’d bet that has to do with every layer – recruitment of appropriate subjects, trial administration, etc:
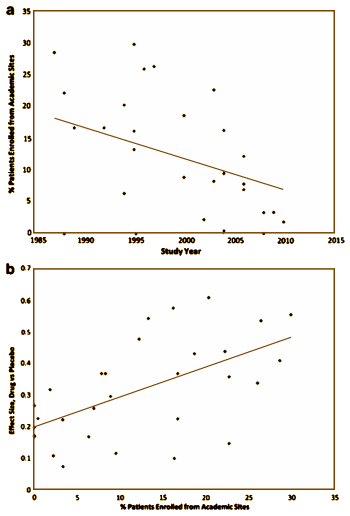
I noticed that non-us trials got done a lot more quickly some time back [the clinical research industry: an invisible empire…]. But I didn’t have the data to look at the degradation in quality overall from the CRO run trials using private CRCs – but it seemed likely:
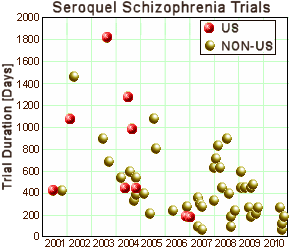
Clinical Trials are probably a necessary evil. If we have to have them, we might as well do them well. What good is a fast, cheap, trial if it’s a bust? Particularly if it’s a bust with a decent drug?
“We don’t,” Frances responds. “And in fact, each expert has the tendency to inflate the part of the elephant (he/she) studies. To me, it is clear the world would be a better place if we were still using the DSM-III.”
NEWS
December 10, 2012
The perils of diagnostic inflation
http://www.cmaj.ca/site/earlyreleases/10dec12_the-perils-of-diagnostic-inflation.xhtml
Canadian Medical Association
Why would they need to do the job well when pharma can just market the ever-loving shit out of it using juked stats and hand-waving? much better ROI that way.
Truly. Pharma marketing spending is much larger than its R&D spending.
I cannot believe the roll-on testosterone ad I saw recently on TV. Mass marketing roll-on steroids? Why the heck not? Pharma doesn’t pay for cancer etc. treatment.
Since the male part of the market is resistant to antidepressants as they are notorious for sexual dysfunction, they’ve been marketed to women. Half the population is underserved by unnecessary medications. Being susceptible to sale pitches involving enhanced sexual potency, guys will be pestering their doctors for roll-on testosterone. Docs will write the scripts without even looking at approved indications, side effects, black box warnings, etc.
No problem, it’s business, Jake.