| While there’s more to say about the Sertraline study mentioned in the last post, specifically about the article version published in 1995, I think I’ll first mention the remaining data available in the N.D.A. that was approved in 1991. Recall the F.D.A. reviewer’s comment: |
 |
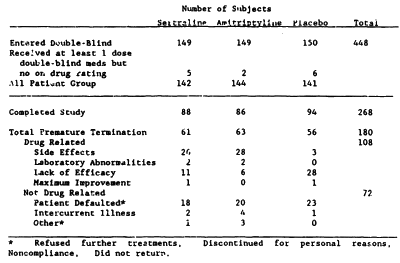
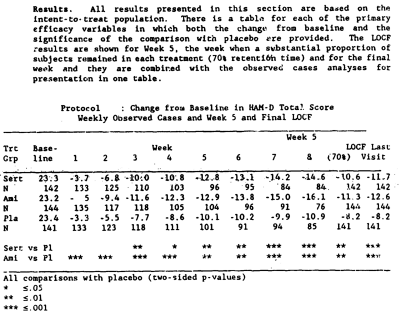
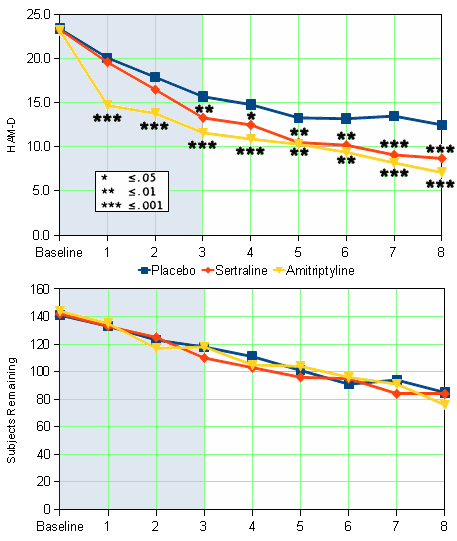
Pfizer submitted its new drug application ["NDA"] to the FDA in 1990. As part of the application, six placebo controlled trials were presented to the FDA. Ofthe six clinical trials, four showed that Zoloft was no more effective than placebo in treating depression and two indicated that Zoloft had slight positive impact on depression. The two studies that showed that Zoloft was more effective than placebo in treating depression, however, were severely flawed.In the second trial that supposedly demonstrated efficacy, researchers enrolled 448 patients in a double-blind trial and divided the patients into three groups- patients taking Zoloft at doses between 50-200mg, patients taking a different anti- depressant, and patients taking placebo. Much like the first "efficacy-establishing" clinical study, approximately 43% of people in the Zoloft treatment groups quit, 18% because of side effects, 9% because of a lack of efficacy, and 16% for other reasons. Of the remaining patients, the researchers tracked HAM-D scale changes over eight weeks. The trial’s data indicated that there was no clinically significant difference between oloft for the first six weeks of treatment, and that in weeks 7 and 8, a person taking Zoloft had a HAM-D scale improvement of about 3.5 points above those taking placebo. This was, again, a very small treatment effect, especially when one considers the serious potential side-effects attendant to Zoloft.
Antidepressant efficacy of sertraline: a double-blind, placebo- and amitriptyline-controlled, multicenter comparison study in outpatients with major depression.
by Reimherr FW, Chouinard G, Cohn CK, Cole JO, Itil TM, LaPierre YD, Masco HL, and Mendels J.
Journal of Clinical Psychiatry. 1990 51 Supplement B:18-27.
A double-blind, placebo- and amitriptyline-controlled comparison study was performed to evaluate the antidepressant efficacy of sertraline, a specific serotonin uptake inhibitor. Patients with DSM-III-defined major depression randomly received either sertraline [N = 149], amitriptyline [N = 149], or placebo [N = 150] once daily for the 8-week study period. The mean final daily medication dose for the all-patients group was 145 mg and 104 mg for the sertraline- and amitriptyline-treatment groups, respectively. As measured by the Hamilton Rating Scale for Depression and the Clinical Global Impressions Scale, both the sertraline and amitriptyline treatment groups showed a significantly greater improvement from baseline [p less than or equal to .001] than the placebo group. The sertraline group had a higher proportion of gastrointestinal complaints and male sexual dysfunction than either the amitriptyline or the placebo group. The amitriptyline group showed a higher proportion of anticholinergic and sedative side effects and dizziness compared with patients who received either sertraline or placebo.
While I have no access to the full published article on this study, the F.D.A. N.D.A. information on this Trial is more convincing than the first one. I can’t argue that a 3.+ point difference in HAM-D isn’t much to write home about, but by F.D.A. standards, this appears to be a significant study.
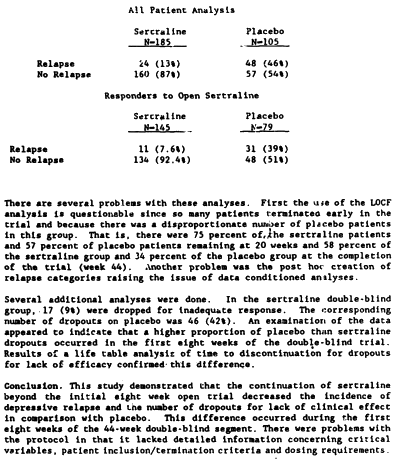
There was a third supportive Clinical Trial reported in the F.D.A. N.D.A. It was a Pfizer conducted study looking at Sertraline in preventing relapse. Patients took Sertraline [open-label] for 8 weeks. Responders were then randomized [Placebo, Sertraline] and followed for 44 weeks. The Baum Hedlund Lawsuit didn’t mention it; the F.D.A. reviewer didn’t know what to say about it; and I’m similarly afflicted – who knows? Here’s the reason for the confusion – their flow chart:
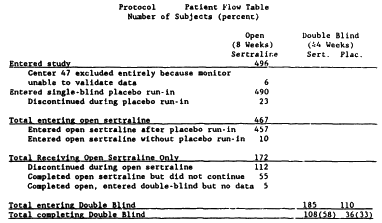
Frankly, this was one of those studies you wish wasn’t there. There’s something a bit off at every level, but you can neither refute nor confirm its results. Here’s what the F.D.A. reviewer reported:
It was published twice:
Sertraline in the prevention of depression.
by Doogan DP and Caillard V
Department of Clinical Research, Pfizer Ltd, Sandwich, Kent.
British Journal of Psychiatry. 1992 160:217-222.
A group of 480 patients, aged 19-78 with an HRSD score of at least 17 and who met DSM-III criteria for major depressive disorder were studied. Patients were given placebo for a one-week single-blind run-in period, after which sertraline was administered for eight weeks. This was followed by 44 weeks in which patients received sertraline or placebo on a double-blind, randomised basis. Patients were assessed periodically using the 17-item HRSD and the Clinical Global Impression scales. During the entire double-blind period 24 [13.0%] sertraline patients relapsed compared with 48 [45.7%] placebo patients [P<0.001]. The protective effect of sertraline was maintained throughout the 44 weeks. The study provides evidence that sertraline prevents relapse of the index episode of depression as well as recurrence of further episodes and has few side-effects.
The influence of different relapse criteria on the assessment of long-term efficacy of sertraline.
by Montgomery SA, Doogan DP, and Burnside R
International Clinical Psychopharmacology. 1991 Supplement 2:37-46.
The treatment of depression with antidepressant agents must be continued beyond the acute phase, until the response is complete. The precise length of this continuation phase is still debated, but most authors estimate that it should last for between 4-6 months after apparent recovery. If antidepressants are withdrawn sooner, the original depression will return [relapse] in a proportion of patients. Relapse rates on placebo are high, whether patients are first-time or recurrent depressives. Most depressions are recurrent and long-term treatment therefore ensures that the changes of a new episode of illness developing are reduced. The importance of this aspect of efficacy is recognized and new antidepressants are being tested in long-term prophylactic studies. A long-term efficacy study has shown that sertraline was significantly more effective than placebo in preventing both relapse and recurrence.
There were just too many drop-outs and the response criteria were too fuzzy to really know what to do with it. The Pfizer authors concluded that it confirmed relapse prevention by Zoloft. I classify it as "whatever."
So here’s what they had. I must add, parenthetically, that, as always, I was impressed with the quality of the report from the F.D.A. reviewer. The reviewers are tasked with summarizing and evaluating the submitted information for the actual "approvers", and they are regularly thorough. Here’s what they had:
| Placebo Controlled Clinical Trials
|
||||||
| Study
|
Site
|
Type
|
Outcome
|
|||
| protocol 103 |
outpatient | fixed dose | questionable | |||
| protocol 101 |
inpatient | fixed dose |
negative | |||
| protocol 310 |
inpatient | fixed dose |
negative | |||
| protocol 104 |
outpatient | titrated dose |
positive | |||
| protocol 315 |
outpatient | titrated dose |
negative | |||
| protocol 320 |
outpatient | open label, relapse | whatever | |||
| So this report went to the approval group, and what we know is that they approved Zoloft in November 1991. But the Baum Hedlund complaint has some actual information about how that came to be from discovery documents collected in the process of filing their suit. I don’t have those documents, but there’s a lot from them in the Laura A. Plumlee et. al. v. Pfizer complaint. That’s where we’re headed next… |

This link may be of interest given the financial incentives in marketing this drug:
http://pharmagossip.blogspot.com/2013/02/rosenberg-and-pringle-what-team.html?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+blogspot%2FDlJuM+%28PharmaGossip%29
Steve Lucas
How can a study with such a small number of people be considered valid? Really, the FDA should at least insist that the study be replicated by two independent sources before giving approval. Just going by the word of drug company funded research is in no way vigilant. In the long run, the government paying for those independent studies would cost far less than the costs imposed by harmful drugs and over-zealous promotion limited only by the drug companies’ imagination.
Can you imagine all those folk that have been duped into believing that Zoloft helped lift their depression when in actual fact it was a placebo effect all along?
Thing is, they will still firmly believe Zoloft helped them because they want to believe they were ill in the first place. Pharma have tapped into this, they reaffirm the ‘illness’ because it helps promote their wares via word of mouth.
Genius marketing that costs very little.
It’s interesting to note that in the 1990 publication by Reimherr et al that you discuss, sertraline (Zoloft) was termed a specific serotonin uptake inhibitor. In a 1988 report Reimherr used the term selective inhibitor of serotonin uptake (PubMed ID 3290941). The term that finally crystallized – selective serotonin reuptake inhibitor or SSRI – as a branding label for this class was invented later. Ironically, the selectivity of action was subtly marketed as a plus, whereas many clinical pharmacologists would have said that the absence of an action on norepinephrine reuptake (which the tricyclic antidepressants possessed) is a net negative for the SSRI class. To this day, that is considered one of the reasons for the many challenges to the efficacy of SSRIs.