| Well here we are at the part of the story we now know all too well. Maybe we don’t know the Pfizer/Zoloft version so well as some of the others, but the involvement of the professional ghost writing firms was apparently an industry-wide story of a literature filled with ghost-written studies with "guest authors." And no, the ghosts aren’t there to make up for the grammatical and stylistic deficits of a bunch of scientists who spent too much time in the chemistry lab and never learned how to put pen to paper. They’re there to play with the numbers and statistics that are supposed to tell us what happened when the volunteers took some unknown medicines, and make them tell us something they weren’t meant to say – something a drug company wants them to say. I had no clue at the time. I doubt that many of us did. And Zoloft was an early drug, so it came before we got rigorous about funding sources, authorship, and conflicts of interest. So these articles looked for all the world like they were legitimate to the journal’s readers unless they were read suspiciously. But they weren’t legitimate – not even close… |
Pfizer understood that the best way to ensure the success of Zoloft was to convince the scientific and medical community that Zoloft was safe and effective by cultivating a body of "peer-reviewed research" to enhance Zoloft’s credibility. To that end, Pfizer created a large-scale ghostwriting program. Pfizer would author, or have a medical communications company author, a study specifically designed to promote a marketing message, i.e., Zoloft’s efficacy. Then, Pfizer would pay "key opinion leaders" ["KOL’ s"] to put their name on the article and get the article published in specifically targeted medical journals. When the article appeared in the journal, there would be no indication of Pfizer’s involvement.
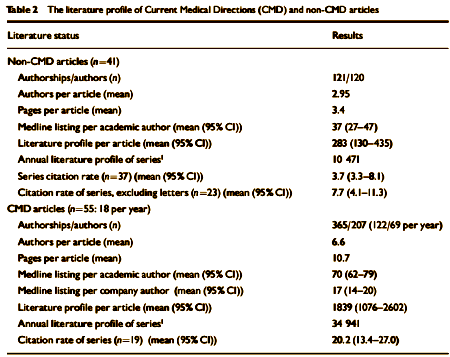
Pfizer had an entire team devoted to the publication of positive medical journal articles about Zoloft. In addition, Pfizer worked with outside medical ghostwriting vendors to create a steady stream of Zoloft-positive medical journal articles. Dr. David Healy, a psychopharmacologist and professor at the University of Wales College of Medicine, and a colleague conducted an analysis of Zoloft articles that were "coordinated" by a medical communications company called Current Medical Direction ["CMD"]. Pfizer had hired CMD to promote Zoloft in the 1990’s. David Healy & Dinah Cattell, Interface Between Authorship, Industry and Science in the Domain of Therapeutics [see below]. According to the study, CMD coordinated over 85 medical journal articles about Zoloft during a three-year period. By early 2001, 55 of these articles had been published in journals such as the New England Journal of Medicine, Journal of the American Medical Association [JAMA], Archives of General Psychiatry and the American Journal of Psychiatry.
Interestingly, all of the clinical trial results were favorable to Zoloft. The analysis found that "the CMD-linked articles reported universally positive results" and that there were "significant discrepancies between published data and the raw data from the actual clinical trials." Most of the 85 articles appeared to have been authored by CMD and, in a number of instances, the authors were listed in Pfizer internal memos as "TBD" [i.e., "to be determined"]. The study concluded that:
The combination of distinguished journal, distinguished author, an efficient distribution system and sponsored platforms appears to have led to an impact on the therapeutics domain greatly in excess of 50% of the impact of the rest of the literature on sertraline. The impact of this literature on third-party payers and other interested parties is at present unquantifiable. The question of literature impact would seem to be tied closely to the nature of ghostwriting. Authorship lines from perceived opinion-leaders with minimal company representation and nondeclaration of other non-academic authorship inputs increase the likelihood that these articles will be influential with prescribers and purchasers.Corroborating the study’s finding, an internal company document shows that CMD kept a "Zoloft publications scorecard" for Pfizer which contained a running list of in-progress medical journal articles with details of the status, the names of the designated authors ["KOLs" or "thought leaders" in their respective fields], and the ghostwriting vendor to be used. An internal PowerPoint presentation prepared by Pfizer in 2000, states that the purpose of the publications program was to, among other things, "promote efficacy," "highlight drug’s superiority to a competitors," "leverage good will with academic investigatorsp" "increase media and public perception of the drug and Pfizer," and "provide tools for sales force to drive prescriptions based on data." The PowerPoint explains that the "bottom line" in publication projects is to "optimize our ability to sell Zoloft."
Interface between authorship, industry and science in the domain of therapeutics
by Healy D and Cattell D.
British Journal of Psychiatry. 2003 183:22-27.
[full text on-line]
BACKGROUND: Changes in the character of medical authorship. Aims To compare the impact of industry-linked and non-industry linked articles.METHOD: We compared articles on sertraline being coordinated by a medical writing agency with articles not coordinated in this way. We calculated numbers of Medline-listed articles per author, journal impact factors, literature profiles and citation rates of both sets of articles.RESULTS: Non-agency-linked articles on sertraline had an average of 2.95 authors per article, a mean length of 3.4 pages, a mean Medline listing of 37 articles per author [95% CI 27-47] and a mean literature profile of 283 per article [95% CI 130-435]. Agency-linked articles on sertraline had an average of 6.6 authors per article, a mean length of 10.7 pages, a mean Medline listing of 70 articles per author [95% CI 62-79] and a mean literature profile of 1839 per article [95% CI 1076-2602]. The citation rate for agency articles was 20.2 [95% CI 13.4-27.0] and for non-agency articles it was 3.7 [95% CI 3.3-8.1].CONCLUSIONS: The literature profiles and citation rates of industry-linked and non-industry-linked articles differ. The emerging style of authorship in industry-linked articles can deliver good-quality articles, but it raises concerns for the scientific base of therapeutics.
The second issue relates to the correspondence between published articles and raw data. The current CMD series throws up issues of concern in this area. First, one study in this series had one patient on sertraline who committed suicide, and three others on sertraline who reported increasing suicidal ideation necessitating treatment discontinuation, in contrast to just one case of emergent suicidality on a comparable drug and no problems on placebo. There is no reference to these data in the final published article. Second, of the six published paediatric psychopharmacology CMD articles, only one article mentions one suicidal act. There were in fact six suicidal acts on sertraline and three further cases of suicidality in the subject group from which these articles come, including four suicidal acts in 44 patients with depression given sertraline, which is a rate of 9% [Pfizer Expert Report, 1997]. The effects of sertraline in paediatric depression were outlined by Alderman et al [1998], who reported only the adverse events that occurred in more than 10% of patients.
How did they land on eleven days? Probably by running the data through SAS over and over to find the optimum interval. I doubt that this old man can think of any eloquent words on an early morning that might stop that kind of playing around with the data. If they didn’t do it, there wouldn’t even be a medical communications company so aptly named, Current Medical Direction. And I don’t know if you noticed, but in the language of the label, Study 2 [Sertraline Protocol 103] still carried the 1991 conclusion, "Study 2 was not readily interpretable regarding a dose response relationship for effectiveness." But by the time it reached the literature in 1995, that got changed into "The results of this study show that sertraline 50 mg once daily is as effective as higher dosages for the treatment of major depression with fewer side effects and therapy discontinuations."
Does the F.D.A. got the raw data? I kind of doubt it, because the reviewer was complaining about some of the correction methods [LOCF]. But what they did get is a lot closer than what we saw in the literature. In the submitted journal articles, the ghost writers have more room to play around – constrained only by the reviewers ability to catch distortions. The most that can happen is the article gets rejected and they move on to another journal. In either the case of the F.D.A. N.D.A. or the journal, all the reviewers have to go on is the submitted material. And the journal’s audience is the most vulnerable of all, counting on the peer reviewers to certify the articles’ contents. So the ghost writer has a lot of wiggle room to spin the story, and in the case of Zoloft, Current Medical Direction had themselves a field day.
| What comes next? What’s the result of all that hard work? Sales of Zoloft was the real outcome parameter ["optimize our ability to sell Zoloft"]. |
Thank you, dr Mickey, for demonstrating the absolute lack of integrity involved in the process of getting the most lucrative compounds, called anti-depressants, of the modern witch-doctor-industry on the world market. They are “blue prescriptions” in this country, meaning paid for by the public purse after a certain amount is reached, strengthening the business rationale of selling depression as a chronic condition, in need of life long medication.
Science it is not, just scams undermining trust in medicine as science, undermining trust in doctors, when so many are seen selling their integrity to the bidders, undermining trust in governments’ ability and willingness to protect their citizens as patients, honest doctors and honest scientists.
So this global scam is – appropriately – under attack by private citizens in US-court rooms and boring, blogging US-doctors. Well done! Keep it up. We need you.
So where are you going with your blog now? You are going to demonize every antidepressant one by one, illustrating every single company had a completely inappropriate and disruptive product that has decimated America?
I have been practicing for 20 years now, every drug has strengths and weaknesses, no argument there, and the push to medicate all of them at high dosages, completely lame to believe. But, Zoloft has not been the demon that Paxil, Lexapro, and to some degree Prozac have been. Don’t know what will be accomplished in just saying Zoloft is as inappropriate as others, but, hope it benefits the site.
Oh, and what would be nice to see retrospectively is how many negative outcomes with antidepressants as a group involved people who had no involvement with psychotherapy. That would speak volumes for me!
Theres no bad drug Joel. No demons chemicals. But there are plenty of lying-ass drug manufaturers. That’s where I’m going. “Don’t know what will be accomplished in just saying Zoloft is as inappropriate as others.” My point is that unless they let us decide for ourselves and our patients instead of hand us a bill of goods, all we can do is guess.
One great step that could be taken forward, is for the limits of the medications to be acknowledged, so that if a prescribed medication isn’t working, a patient can be told simply that it isn’t working and recommend higher or lower doses, or trying something else instead of labeling the patient as “treatment resistant” as if they have a most virulent form of depression.
Joel, under what circumstances would you prescribe Zoloft now you know this information?
First and foremost, as an ADJUNCT to therapy, because for those who have gone to my own blog, it is about therapy first, and you CAN’T MEDICATE LIFE which is what a good deal of depression is as a reaction. And, having prescribed Zoloft fairly much since I finished residency in 1993, it is the most tolerated SSRI of all of them, see Celexa as a close second, and the key is titrating and keeping the effective dose as low as effective, preferably at or below 100mg as a target.
I do agree with Mickey, all the pharma companies are complicit with greed and profit first, patient efficacy second or further down the list per benefiting associated cronies as able. Let’s face it, I know that many commenters here, and this is NOT targeted to you specifically Altostrata, but, they are just focusing on the negativity of psychotropics at large that has to be maintained. But, I have seen many patients make much momentous and effective strides by being on medication and participating in therapy, AND, later get off the meds and continue to do well!
Yes, I come from the school of thought that meds are a temporary part of care until proven otherwise. But, in 2013, how many clinicians and patients want to hear much less think this!? In the end, your audience by in large is not interested in the truth, but, what are the easy outs and maintaining that dependency?
That is my post tonight at my own site, if interested.