 The STAR*D study came to us from the dawn of a new century – an outgrowth of a notion that one could improve the effectiveness of the antidepressants by creating an algorithm for trying different drugs when there was a treatment failure [Sequenced Treatment Alternatives to Relieve Depression]. It had already been implemented in Texas as TMAP [Texas Medical Algorithm Project] and it was a natural for the highest ticket NIMH grant in history [a thirty-five million dollar misunderstanding…]. The results finally reported in 2006:
The STAR*D study came to us from the dawn of a new century – an outgrowth of a notion that one could improve the effectiveness of the antidepressants by creating an algorithm for trying different drugs when there was a treatment failure [Sequenced Treatment Alternatives to Relieve Depression]. It had already been implemented in Texas as TMAP [Texas Medical Algorithm Project] and it was a natural for the highest ticket NIMH grant in history [a thirty-five million dollar misunderstanding…]. The results finally reported in 2006: Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report.
by Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M.
Am J Psychiatry. 2006 163[11]:1905-1917.
OBJECTIVE: This report describes the participants and compares the acute and longer-term treatment outcomes associated with each of four successive steps in the Sequenced Treatment Alternatives to Relieve Depression [STAR*D] trial.CONCLUSIONS: When more treatment steps are required, lower acute remission rates [especially in the third and fourth treatment steps] and higher relapse rates during the follow-up phase are to be expected. Studies to identify the best multistep treatment sequences for individual patients and the development of more broadly effective treatments are needed.
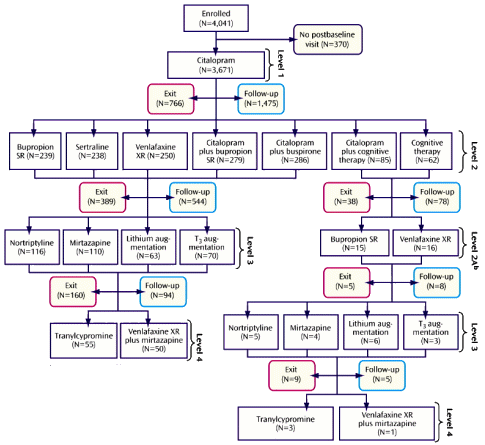
But there was something else. This study that couldn’t bring off collecting or publishing its own primary data continues to be a well-spring for an endless string of publications. PubMed lists 142 articles with STAR*D in the title, but there are many where that phrase isn’t in the title [below]. They’ve had the same editorial·assistant/ghost·writer throughout – Jon Kilner, a science fiction author in Pittsburgh, and many of the papers have the original authors in some reshuffled order.
Increase in Work Productivity of Depressed Individuals With Improvement in Depressive Symptom Severity.
by Trivedi MH, Morris DW, Wisniewski SR, Lesser I, Nierenberg AA, Daly E, Kurian BT, Gaynes BN, Balasubramani GK, and Rush AJ.
American Journal of Psychiatry. 2013 Apr 5.
[Epub ahead of print]
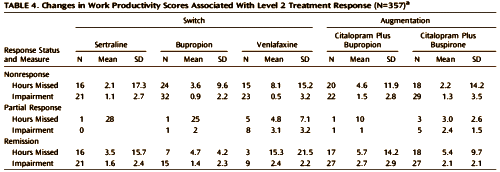
OBJECTIVE: The authors sought to identify baseline clinical and sociodemographic characteristics associated with work productivity in depressed outpatients and to assess the effect of treatment on work productivity.METHOD: Employed depressed outpatients 18-75 years old who completed the Work Productivity and Activity Impairment scale [N=1,928] were treated with citalopram [20-40 mg/day] in the Sequenced Treatment Alternatives to Relieve Depression study. For patients who did not remit after an initial adequate antidepressant trial [level 1], either a switch to sertraline, sustained-release bupropion, or extended-release venlafaxine or an augmentation with sustained-release bupropion or buspirone was provided [level 2]. Participants’ clinical and demographic characteristics and treatment outcomes were analyzed for associations with baseline work productivity and change in productivity over time.RESULTS: Education, baseline depression severity, and melancholic, atypical, and recurrent depression subtypes were all independently associated with lower benefit to work productivity domains. During level 1 treatment, work productivity in several domains improved with reductions in depressive symptom severity. However, these findings did not hold true for level 2 outcomes; there was no significant association between treatment response and reduction in work impairment. Results were largely confirmed when multiple imputations were employed to address missing data. During this additional analysis, an association was also observed between greater impairment in work productivity and higher levels of anxious depression.CONCLUSIONS: Patients with clinically significant reductions in symptom severity during initial treatment were more likely than nonresponders to experience significant improvements in work productivity. In contrast, patients who achieved symptom remission in second-step treatment continued to have impairment at work. Patients who have demonstrated some degree of treatment resistance are more prone to persistent impairment in occupational productivity, implying a need for additional, possibly novel, treatments.
2006: "Dr. Trivedi has served as an advisor, consultant, or speaker for or received research support from Abbott Laboratories, Inc.; Akzo [Organon Pharmaceuticals]; Bayer; Bristol-Myers Squibb Company; Cephalon, Inc.; Corcept Therapeutics, Inc.; Cyberonics, Inc.; Eli Lilly; Forest Pharmaceuticals; GlaxoSmithKline; Janssen Pharmaceutica; Johnson & Johnson PRD; Meade Johnson; the National Institute of Mental Health; the National Alliance for Research in Schizophrenia and Depression; Novartis; Parke-Davis Pharmaceuticals, Inc.; Pfizer Inc; Pharmacia & Upjohn; Predix Pharmaceuticals; Sepracor; Solvay Pharmaceuticals, Inc.; and Wyeth-Ayerst Laboratories."
2013: "Dr. Trivedi has received research support from or served as an adviser, consultant, or speaker for Abbott Laboratories, Abdi Ibrahim, Agency for Healthcare Research and Quality, Akzo [Organon Pharmaceuticals], Alkermes, AstraZeneca, Axon Advisors, Bristol-Myers Squibb, Cephalon, Corcept Therapeutics, Cyberonics, Eli Lilly, Evotec, Fabre Kramer Pharmaceuticals, Forest Pharmaceuticals, Glaxo-SmithKline, Janssen Pharmaceutica Products, Johnson & Johnson PRD, Libby, Lundbeck, Mead Johnson, MedAvante, Medtronic, Merck, National Institute on Drug Abuse, NARSAD, Naurex, Neuronetics, NIMH, Novartis, Otsuka Pharmaceuticals, Pamlab, Parke-Davis Phar-maceuticals, Pfizer, PgxHealth, Pharmacia & Upjohn, Predix Pharmaceuticals [Epix], Rexahn Pharmaceuticals, Roche Products, Sepracor, Shire Development, Sierra, SK Life and Science, Solvay Pharmaceuticals, Takeda, Transcept, VantagePoint, and Wyeth-Ayerst Laboratories."
 Long ago, I had a beagle who loved to chase tennis balls. I tried for years to beat him, throw the balls longer than he was willing to chase them. I never won the game, not once. I finally stopped when he got really old because I was afraid he’d die running after the balls. I feel the same way about these STAR*D articles – that they’ll keep coming long after I’m gone. I guess I see them as a testimonial to the lowest of points in our history. Why they continue to be written, or for that matter, published, is beyond my understanding…
Long ago, I had a beagle who loved to chase tennis balls. I tried for years to beat him, throw the balls longer than he was willing to chase them. I never won the game, not once. I finally stopped when he got really old because I was afraid he’d die running after the balls. I feel the same way about these STAR*D articles – that they’ll keep coming long after I’m gone. I guess I see them as a testimonial to the lowest of points in our history. Why they continue to be written, or for that matter, published, is beyond my understanding…
fyi
http://content.healthaffairs.org/content/early/2013/04/22/hlthaff.2011.0596
Independent Review Of Social And Population Variation In Mental Health Could Improve Diagnosis In DSM Revisions
Helena B. Hansen1,*,
Zoe Donaldson2,
Bruce G. Link3,
Peter S. Bearman4,
Kim Hopper5,
Lisa M. Bates6,
Keely Cheslack-Postava7,
Kristin Harper8,
Seth M. Holmes9,
Gina Lovasi10,
Kristen W. Springer11 and
Julien O. Teitler12
+ Author Affiliations
1Helena B. Hansen (helena.hansen@nyumc.org) is an assistant professor of psychiatry and anthropology at New York University, in New York City.
2Zoe Donaldson is a postdoctoral researcher at Columbia University, in New York City.
3Bruce G. Link is a professor of epidemiology at Columbia University.
4Peter S. Bearman is the Cole Professor of Social Science at Columbia University.
5Kim Hopper is a professor at the Mailman School of Public Health, Columbia University.
6Lisa M. Bates is an assistant professor in the Department of Epidemiology and the Department of Population and Family Health at Columbia University.
7Keely Cheslack-Postava is a psychiatric epidemiology postdoctoral fellow at Columbia University.
8Kristin Harper is a cancer epidemiology fellow in the Department of Environmental Health Sciences at Columbia University.
9Seth M. Holmes is the Martin Sisters Endowed Chair Assistant Professor in the School of Public Health and the Graduate Program in Medical Anthropology at the University of California, Berkeley.
10Gina Lovasi is an assistant professor at the Mailman School of Public Health, Columbia University.
11Kristen W. Springer is an associate professor of sociology at Rutgers University, in New Brunswick, New Jersey.
12Julien O. Teitler is an associate professor of social work and sociology at Columbia University.
?*Corresponding author
Abstract
At stake in the May 2013 publication of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), are billions of dollars in insurance payments and government resources, as well as the diagnoses and treatment of millions of patients. We argue that the most recent revision process has missed social determinants of mental health disorders and their diagnosis: environmental factors triggering biological responses that manifest themselves in behavior; differing cultural perceptions about what is normal and what is abnormal behavior; and institutional pressures related to such matters as insurance reimbursements, disability benefits, and pharmaceutical marketing. In addition, the experts charged with revising the DSM lack a systematic way to take population-level variations in diagnoses into account. To address these problems, we propose the creation of an independent research review body that would monitor variations in diagnostic patterns, inform future DSM revisions, identify needed changes in mental health policy and practice, and recommend new avenues of research. Drawing on the best available knowledge, the review body would make possible more precise and equitable psychiatric diagnoses and interventions.
full text of above article is available on line
Just curious to readers with an opinion to this matter, why did the STAR*D use a max 60mg dose with Citalopram that is now considered inappropriate by the FDA?
And why is Lexapro NOT considered an equal risk factor with equivalent dosage per that purified form?
Will be back in 3-4 days to see if readers respond.
Joel H
Ha. The first thing I thought of when I saw “TMAP” was a girl being put on 12 different medications. It took less than a second to find the story in Mother Jones
The family would not be allowed to see or even speak to their daughter for the next five months, and Amanda would spend a total of nine months in a state psychiatric hospital and residential treatment facilities. While in the hospital, she was placed in restraints more than 26 times and medicated—against her will and without her parents’ consent—with at least 12 different psychiatric drugs, many of them simultaneously.
It’s not too much of a stretch to think that by having the girl and her parents meet with a child psychologist or family therapist with the understanding that the school was not going to tolerate her bad behavior that this little problem could have been worked out without a diagnosis, drugs, institutionalization, confinement, and restraints.
Dear God, pity the parents and children who are/were put through this psychological and physical torture over a behavioral problem that likely could have been “cured” in six weeks, or less of negotiation and determined action to hold all involved accountable.
Do these pediatric psychiatric researchers remember being a child? How they thought? How they felt? What kinds of problems they had?
jamzo, thanks for that link. How our society defines mental health and illness is really too important to be left to a select group within the APA.
Dr. Hassman, I think at the time of the STAR*D trial, it was fairly common for practitioners to use citalopram at 60mg; the FDA warning on citalopram did not come out until August 2011. According to this UK government site, escitalopram also shows a dose-dependent increase in QTc, but less so than with citalopram.
Regarding citalopram, I believe clinical trials also showed no increased benefit of a 60mg dose over a 40mg dose — yet the dosage was poured on anyway, as is typical in psychiatry.
Let us not forget, the STAR*D-related studies also invariably demonstrate the superiority of treatment with antidepressants, the more the better. “The inevitable recommendation for further study” is merely window-dressing to make sheer propaganda look like open-minded scientific inquiry.