In the story of every tension, there’s a time for reflection and understanding, and then there’s a time for action. But what action? The strange professor in my World Religion I Course had a lecture he’d given countless times, always with the same passion as the first day he wrote it. "Primitive man," he said, "had only two paths to follow in the face of an inhospitable nature." After a long pause, he continued, "Pious Petition – praying to the universe for mercy, And…" After another pause, he adopted an impish grin, "Magic! – trying to force the universe into compliance." He went on to talk about how the former became religion and the latter was the precursor to science [the pity was, that first lecture kept us from dropping the course, but the following months could only be called soporific].
The time for just decrying the shameful abuse of clinical trials in psychiatry and the rest of medicine has passed. The books and blogs are joined in a monotonous lamentation of the way clinical trials have been conducted. Prophetically, the first major action with traction was a petition – AllTrials – not totally pious, but a request nonetheless. In the background, the magicians have been stirring. The Cochrane Collaboration, Peter Doshi, and Tom Jefferson have been playing hard chess with Roche around the billion dollar efficacy questions with Tamiflu. Healthy Skepticism and others have dogged the JAACAP over Paxil Study 329 for a decade. Dr. David Healy’s Pharmageddon and Rxisk database have taken on trials as well as adverse effects in general. Comes now RIAT [Restoring Invisible and Abandoned Trials] backed by a broad collaboration proposing a plan to add some teeth to the demands for clinical trial reform, focusing on missing and jury-rigged studies. The plan was announced today by the British Medical Journal and PLoS Medicine:
| BMJ Press Release
Experts propose restoring invisible and abandoned trials “to correct the scientific record” |
|
Experts are today calling for all unpublished and misreported trials to be published or formally corrected within the next year to ensure doctors and patients rely on complete and accurate information to make decisions about treatments.
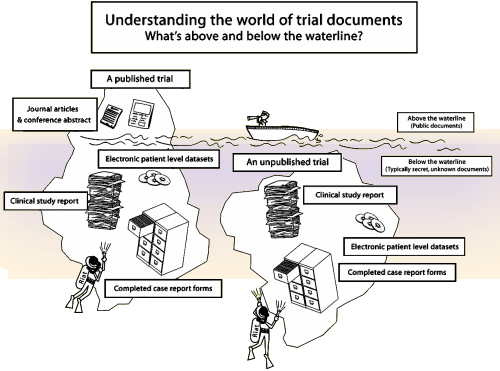
Sponsors and researchers will be given one year to act before independent scientists begin publishing the results themselves using previously confidential trial documents. The BMJ and PLOS Medicine have already endorsed the proposal and committed to publishing restorative clinical trial submissions – and will discuss it in more detail at a meeting in London on Friday 14 June 2013. Unpublished and misreported studies make it difficult to determine the true value of a treatment. Around half of all clinical trials for the medicines we use today have never been published – and a whole range of widely used drugs have been represented as safer and more effective than they are, putting patients at risk and wasting public money. The authors of the declaration, led by Peter Doshi, a postdoctoral fellow at Johns Hopkins University School of Medicine, will contact manufacturers of trials, asking them to signal their intent within 30 days to publish previously unpublished trials and formally correct previously misreported trials (i.e. to restore abandoned trials). They propose that if anyone who declares an intention to publish or correct does not do so within one year, all publicly available data for such trials should be considered “public access data” that others are allowed to publish. This declaration, they say, “offers sponsors and trialists an opportunity to publish or formally correct their studies” – or otherwise see those abandoned studies published or republished by others. New freedom of information policies means the public and the authors have access to around 178,000 pages of previously confidential trial documents and clinical study reports for widely used drugs for depression, heart disease, epilepsy and influenza. Some trials remain unpublished years after completion, while others have been published but have been shown to contain inaccuracies. They say they are committed to seeing the findings from abandoned trials published – and misreported trials corrected and republished – and they set out a method for responsibly restoring invisible and abandoned trials (RIAT). “We see RIAT as a collaborative, global effort, and over the next year we hope to discuss and debate our proposal at appropriate venues,” they write. As such, they call on others to join them as volunteers “in place of those who should have but did not make trial reports visible and accessible.” And they ask medical journal editors to endorse the concept of restorative authorship to “help the effort to complete and correct the scientific record.” In an accompanying editorial, editors at The BMJ and PLOS Medicine say Doshi and colleagues “offer a bold remedy” to help restore the integrity of the clinical trial evidence base. They explain that the results of clinical trials “are a public, not a private, good” and that the public interest “requires that we have a complete view of previously conducted trials and a mechanism to correct the record for inaccurately or unreported trials.” They conclude: “If we do not act on this opportunity to refurbish and restore abandoned trials, the medical research community will be failing its moral pact with research participants, patients, and the public. It is time to move from whether to how, and from words to action.”
|
Restoring invisible and abandoned trials: a call for people to publish the findings
Analysis
British Medical Journal
by Peter Doshi, Kay Dickersin, David Healy, S Swaroop Vedula, and Tom Jefferson
June 13, 2013
[full text on-line]
Restoring the integrity of the clinical trial evidence base
Editorial
British Medical Journal
by Elizabeth Loder and Fiona Godlee [BMJ] and Virginia Barbour and Margaret Winker [PLoS Medicine]
June 13, 2013
[full text on-line]
Restoring Invisible and Abandoned Trials: A Creative Approach to a Public Good; Now a Creative Approach to Implementation is Needed
Blog
PLoS Medicine
By Margaret Winker and Virginia Barbour
June 13, 2013
[full text on-line]
Academic freedom is the belief that the freedom of inquiry by faculty members is essential to the mission of the academy as well as the principles of academia, and that scholars should have freedom to teach or communicate ideas or facts [including those that are inconvenient to external political groups or to authorities] without being targeted for repression, job loss, or imprisonment… Academic tenure protects academic freedom by ensuring that teachers can be fired only for causes such as gross professional incompetence or behavior that evokes condemnation from the academic community itself.
The pharmaceutical clinical trials have been accepted into our scientific literature based on the assumption that the academic authors know the rules and are accurately representing the data. But there is widespread evidence that this is an erroneous assumption. Many of the academic authors are compromised by conflicts of interest [or are not even the actual authors]. The traditional counterpose is peer review, but this has been blocked by a firewall with the claim that the raw data is private property.
There are only two solutions to this problem. The first is to deny access to the academic literature to any study withholding public access to the raw data itself. That is the approach taken by the AllTrials petition – an eminently sensible solution. But it doesn’t address the volumes of studies already published, and their authors and sponsors aren’t being forthcoming. I guess they’re claiming the rights of a former loophole.
I had a “strange professor” also – freshman year in college, Sociology 101.
He repeated the same statement each lecture, as if it were ‘Truth’ itself (in the upper case):
“Human behavior is unpredictable.”
I’ve never forgotten those words.
Duane
From Institutional Review Blog:
“As part of the interview, Levine explains why the Common Rule defines “research” in reference to design, rather than intent”
The idea,you can spin the clinical trial to whatever intent by design.
http://www.institutionalreviewblog.com/2013/06/robert-levine-we-should-have-done.html
In other words, those divers might find a subjective projected diagnosis upon patients with predetermined outcomes.
There is a third choice— hit the stacks religiously. Thy rod is determined, thy staff needs no ransom, thy cloak has no guile.
Off-topic:
Wonder what to make of this:
http://www.nature.com/news/no-dishonour-in-depression-1.13170
via pharmagossip.
On the subject of ‘Bold Remedies’ –
The Vatican is taking one with its conference on psychiatric drugs. –
Livestream feed (don’t miss the interview with Dr. Joanna Moncrieff) –
http://new.livestream.com/accounts/3973214/events/2146679
IMO, if the field is not going to make a break from its unholy alliance with pharma, other institutions will do so. This is likely the first of many upcoming dialogues at Vatican, and will set the stage for other religious groups to do the same – protect children and mothers from the dangers of these horrible drugs.
Duane
And the message of their harm will be passed along to adults, leaving *history* to be the final judge of where we went wrong as a society – to believe we could numb our brains into health; medicate our souls into happiness.
Psychiatric drugs cause more harm than good for the vast majority – especially when used long-term. They do not cure chemical imbalance. They cause it.
And they should *not* be prescribed to children.
Duane
FYI
links to two articles discussing changing roles of pharma, payers, clinicians and information technology companies in healthcare…they elucidate the increasing role of information technology in healthcare and the prospects that have been assigned to “big data”…each article is looking at the larger healthcare environment of which mental health
the first article from the financial times speculates what pharma could do if…
Chronic disease conditions could attract pharma-accountable care organization collaboration
http://www.ft.com/intl/cms/s/2/ddd386f0-c172-11e2-b93b-00144feab7de.html#axzz2WCL48BXo
the second article is a post by a regular blogger in the philadelphia inquirer…it deals with what actually is happening not what if…… it discusses how pharma is not ready, willing, or able to adapt to a new role in a changing healthcare environment…
Pharma as a fading power hitter
POSTED: Wednesday, June 12, 2013, 6:00 AM
Daniel R. Hoffman, Ph.D.
Filed Under: Daniel Hoffman
http://www.philly.com/philly/blogs/healthcare/Pharma-as-a-fading-power-hitter.html
A day of hope for medication and science. Thank you Peter Doshi, Kay Dickersin, David Healy, S Swaroop Vedula, and Tom Jefferson, and BMJ and PLoS Medicine. And Mickey for your superb, succinct blogging. I have just posted this wonderful news of an extraordinary initiative to as many Lists and FB groups as I possibly can.
I feel that there has been too much emphasis on the problems with pharmaceutical companies and trials in coverage of these issues, and that this gives the impression that there are not similar problems with research into talking and behavioural interventions. My suspicion is that, as we gain access to more raw data from these trials, particularly those with appropriate trials, we will come to see that much published research has provided an exaggerated view of the efficacy of a very wide range of medical interventions. The pursuit of prestige and attention can be just as corrupting as pharmaceutical profits.
I think that people are going to look back at the way we have been presenting research with a real sense of shame.
The fulcrum:
“Restoring Invisible and Abandoned Trials: A Creative Approach to a Public Good; Now a Creative Approach to Implementation is Needed”
By Margaret Winker and Virginia Barbour
Posted: June 13, 2013
“Ethical oversight: RIAT studies submitted by the original investigators require the same ethical oversight of institutional review board (IRB) approval and patient informed consent as all other studies. However, restorative authors may not have access to all the documentation that the original investigators did. We believe it is essential for studies to have professional ethical oversight. One possibility would be for restorative authors to work with their IRB to have the study protocol reviewed for evidence of ethical lapses. If this review is performed, the IRB review should be included with the submission. If IRBs are not willing or able to do so, we may involve an ethicist in the peer review process to help identify ethical concerns. We are interested in feedback from stakeholders, particularly ethicists and IRBs, as to the feasibility of these proposals.”
http://blogs.plos.org/speakingofmedicine/2013/06/13/restoring-invisible-and-abandoned-trials-a-creative-approach-to-a-public-good-now-a-creative-approach-to-implementation-is-needed/