I said a few days ago that I never met a graph I didn’t like, but there’s been a graph that I’ve wanted to see for years, but I never found the data to draw it. But as of today, I can put all of that behind me:
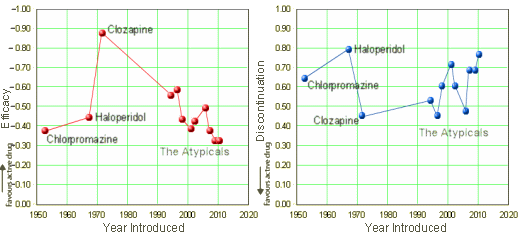
On the left, the Efficacy of the Antipsychotics plotted against the year the drug was introduced [the higher Efficacy is up]. On the right, the discontinuation rate plotted against the year the drug was introduced [the higher discontinuation rate is up]. Why have I wanted to draw this graph? Because it feels like we haven’t gotten very far – and that’s more or less true. Side effect profiles may have changed over the years, but by patient vote [discontinuation rate] there’s not a lot to crow about. A more interesting question is, How did I draw this graph? It’s because the Lancet has published the mother of all meta-analyses of the antipsychotics:
by Prof Stefan Leucht MD, Andrea Cipriani MD, Loukia Spineli, Dimitris Mavridis PhD, Deniz Örey MD, Franziska Richter MD, Myrto Samara MD, Prof Corrado Barbui MD, Prof Rolf R Engel PhD, Prof John R Geddes MD, Werner Kissling MD, Marko Paul Stapf, Bettina Lässig, Georgia Salanti PhD, Prof John M Davis MDThe Lancet, Early Online Publication, 27 June 2013, doi:10.1016/S0140-6736[13]61032-6
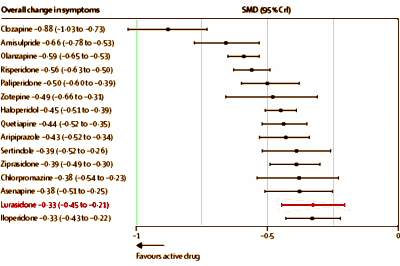
Background: The question of which antipsychotic drug should be preferred for the treatment of schizophrenia is controversial, and conventional pairwise meta-analyses cannot provide a hierarchy based on the randomised evidence. We aimed to integrate the available evidence to create hierarchies of the comparative efficacy, risk of all-cause discontinuation, and major side-effects of antipsychotic drugs.Methods: We did a Bayesian-framework, multiple-treatments meta-analysis [which uses both direct and indirect comparisons] of randomised controlled trials to compare 15 antipsychotic drugs and placebo in the acute treatment of schizophrenia. We searched the Cochrane Schizophrenia Group’s specialised register, Medline, Embase, the Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov for reports published up to Sept 1, 2012. Search results were supplemented by reports from the US Food and Drug Administration website and by data requested from pharmaceutical companies. Blinded, randomised controlled trials of patients with schizophrenia or related disorders were eligible. We excluded trials done in patients with predominant negative symptoms, concomitant medical illness, or treatment resistance, and those done in stable patients. Data for seven outcomes were independently extracted by two reviewers. The primary outcome was efficacy, as measured by mean overall change in symptoms. We also examined all-cause discontinuation, weight gain, extrapyramidal side-effects, prolactin increase, QTc prolongation, and sedation.Findings: We identified 212 suitable trials, with data for 43 049 participants. All drugs were significantly more effective than placebo. The standardised mean differences with 95% credible intervals were: clozapine 0·88, 0·73—1·03; amisulpride 0·66, 0·53—0·78; olanzapine 0·59, 0·53—0·65; risperidone 0·56, 0·50—0·63; paliperidone 0·50, 0·39—0·60; zotepine 0·49, 0·31—0·66; haloperidol 0·45, 0·39—0·51; quetiapine 0·44, 0·35—0·52; aripiprazole 0·43, 0·34—0·52; sertindole 0·39, 0·26—0·52; ziprasidone 0·39, 0·30—0·49; chlorpromazine 0·38, 0·23—0·54; asenapine 0·38, 0·25—0·51; lurasidone 0·33, 0·21—0·45; and iloperidone 0·33, 0·22—0·43. Odds ratios compared with placebo for all-cause discontinuation ranged from 0·43 for the best drug [amisulpride] to 0·80 for the worst drug [haloperidol]; for extrapyramidal side-effects 0·30 [clozapine] to 4·76 [haloperidol]; and for sedation 1·42 [amisulpride] to 8·82 [clozapine]. Standardised mean differences compared with placebo for weight gain varied from −0·09 for the best drug [haloperidol] to −0·74 for the worst drug [olanzapine], for prolactin increase 0·22 [aripiprazole] to −1·30 [paliperidone], and for QTc prolongation 0·10 [lurasidone] to −0·90 [sertindole]. Efficacy outcomes did not change substantially after removal of placebo or haloperidol groups, or when dose, percentage of withdrawals, extent of blinding, pharmaceutical industry sponsorship, study duration, chronicity, and year of publication were accounted for in meta-regressions and sensitivity analyses.Interpretation: Antipsychotics differed substantially in side-effects, and small but robust differences were seen in efficacy. Our findings challenge the straightforward classification of antipsychotics into first-generation and second-generation groupings. Rather, hierarchies in the different domains should help clinicians to adapt the choice of antipsychotic drug to the needs of individual patients. These findings should be considered by mental health policy makers and in the revision of clinical practice guidelines.
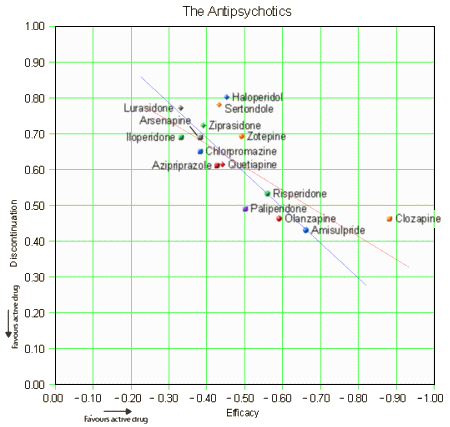
The article remains behind a pay-wall. I would hope that it will become freely available on-line at some point. The forest plot above is for efficacy [as in the abstract], but they have one for each in the article itself: discontinuation, weight gain, EPS, Prolactin, QT prolongation, and sedation. Not satisfied with that, there are tables showing the comparative results in studies with active comparators for these parameters. I had a go at plotting Efficacy against Discontinuation Rate [two linear regressions – with and without Clozapine]. We usually think of discontinuation as being a function of adverse effects, but the regression lines suggest the efficacy is a significant factor:
It’s kind of interesting to think back on my own training in the 1970s. Other than Haldol, the drugs we used then aren’t even on the list: Fluphenazine [Prolixin]; Perphenazine [Trilafon]; Thioridazine [Mellaril]; Trifluoperazine [Stelazine]; Thiothixene [Navane]. In those days, we ruminated the relative merits and profiles of each of those drugs just like I’m sure the recent residents obsess about the Atypicals. Haldol was barely the first-line drug then, but it appears to be the only one that survived to the present. The article itself has a lot of information about the trails themselves [212!]. I report it here to mark that it exists. I think it needs some time to digest before making much in the way of commentary.
A typo: “about the trails themselves”
A question: did they say why they didn’t look at perphenazine given that it got so much press as part of the CATIE study?
I haven’t read more than this post yet but my guess is that the reason no other FGA’s were included is that the efficacy studies on the FGA’s, except for haloperidol, are so old. Haloperidol has been the primary drug used as an active arm in modern studies. Since this data – to the extent I have reviewed it – is consistent with my clinical experience, I suspect perphenazine would fall along these lines with haloperidol and risperidone in terms of both efficacy and discontinuation. One big confounder is dose; haloperidol is often compared at high doses, higher than what is needed to get an optimal response.
Another drug that is missed is molindone which did not cause weight gain but is no longer made.
We are also having problems getting fluphenazine decanoate. Perhaps I am paranoid but it seems like no small coincidence that this is occcuring as there is a push to prescribe the newer – branded – long acting injectables.
What was so frustrating to me in the years that these drugs were first marketed is that the initial studies never demonstrated better efficacy. This meta- analysis is in that way not surprising. It was only in the hype that they were touted as better.
Sandra,
It is common in the drug industry to drop drugs as they come off patent. In some cases a drug company will stop selling a drug a couple of years before it goes off patient and introduce a XR version knowing that many prescriptions will be switched and thus cutting down on the generic market.
Recently we have seen legal cases involving “pay to delay.” Here a drug company will pay a large generic maker a large fee not to introduce a generic equivalent to a drug they have coming off patent.
This link will get you started on the story of Tamiflu:
http://www.margaretsoltan.com/?p=40408
Steve Lucas
Thanks, Steve. I am pretty familiar with much of this and the NYT had a good story yesterday about Tamiflu. I wish I could get the back story on fluphenazine decanoate and haloperidol decanoate. I would not say they are “good” drugs but they are much cheaper and as effective as the newer LAIs.
This is perhaps the most important paper on antipsychotics ever written. Why isn’t it getting more attention in the psychiatry publications??