by William V. Bobo, William O. Cooper, C. Michael Stein, Mark Olfson, David Graham, James Daugherty, Catherine Fuchs, Wayne A. RayJAMA Psychiatry. on-line first. August 21, 2013
Importance The increased prescribing of antipsychotics for children and youth has heightened concerns that this practice increases the risk of type 2 diabetes mellitus.Objective To compare the risk of type 2 diabetes in children and youth 6 to 24 years of age for recent initiators of antipsychotic drugs vs propensity score–matched controls who had recently initiated another psychotropic medication.Design, Setting, and Participants Retrospective cohort study of the Tennessee Medicaid program with 28 858 recent initiators of antipsychotic drugs and 14 429 matched controls. The cohort excluded patients who previously received a diagnosis of diabetes, schizophrenia, or some other condition for which antipsychotics are the only generally recognized therapy.Main Outcomes and Measures Newly diagnosed diabetes during follow-up, as identified from diagnoses and diabetes medication prescriptions.Results Users of antipsychotics had a 3-fold increased risk for type 2 diabetes [HR = 3.03 [95% CI = 1.73-5.32]], which was apparent within the first year of follow-up [HR = 2.49 [95% CI = 1.27-4.88]]. The risk increased with cumulative dose during follow-up, with HRs of 2.13 [95% CI = 1.06-4.27], 3.42 [95% CI = 1.88-6.24], and 5.43 [95% CI = 2.34-12.61] for respective cumulative doses [gram equivalents of chlorpromazine] of more than 5 g, 5 to 99 g, and 100 g or more [P < .04]. The risk remained elevated for up to 1 year following discontinuation of antipsychotic use [HR = 2.57 [95% CI = 1.34-4.91]]. When the cohort was restricted to children 6 to 17 years of age, antipsychotic users had more than a 3-fold increased risk of type 2 diabetes [HR = 3.14 [95% CI = 1.50-6.56]], and the risk increased significantly with increasing cumulative dose [P < .03]. The risk was increased for use restricted to atypical antipsychotics [HR = 2.89 [95% CI = 1.64-5.10]] or to risperidone [HR = 2.20 [95% CI = 1.14-4.26]].Conclusions and Relevance Children and youth prescribed antipsychotics had an increased risk of type 2 diabetes that increased with cumulative dose.
by Jonathan S. Comer, Ramin Mojtabai, and Mark OlfsonAmerican Journal of Psychiatry 2011 168:1057-1065.Conclusions: Although little is known about their effectiveness for anxiety disorders, antipsychotic medications are becoming increasingly prescribed to psychiatric outpatients with these disorders.
It was accompanied with an editorial [see nothing is simple anymore…] by academic Dr. Alan Breier saying we needed "more research" before worrying about such things. Dr. Breier turned out to be an academic only since he left being the chief scientist at Eli Lilly in charge of Zyprexa right before Zyprexa received a then record breaking fine for marketing practices with this drug [Eli Lilly and Company Agrees to Pay $1.415 Billion to Resolve Allegations of Off-label Promotion of Zyprexa].
He freely described his Sales Reps promoting Risperal in children. One strategy was to start with Concerta [McNeil’s ADHD drug] then move to Risperdal – using Concerta to get in the door. He explained, "You can’t be a billion dollar product in a 1% market [Schizophrenia]. The evidence [email chains, Call Notes] showed that the focus was on promoting Risperdal in kids, and went up and down the hierarchy in Janssen’s sales force – from the top dogs to the rep on the calls – in other words, it was corporate policy. Toni’s testimony included other things too. Texas Medicaid was a target ["big payer"]. The reps fought the allegations in the FDA Warning about Diabetes on "every sales call." They were trained on how to sell "off-label" eg, a training slide about promoting Risperdal in kids under 13 said "most diagnosed with behavior disorders or mood disorders", "No Indications!!!", "sell on symptoms not diagnosis". These were not rogue reps, they were trained to carry the message.
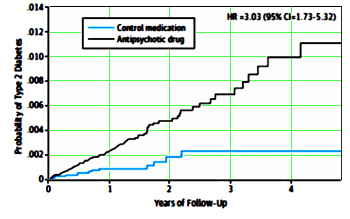
I’m not complaining about this recent article. I appreciate their sticking to the task until they could find a cohort that was available to demonstrate what we already knew a long time ago. This kind of epidemiological research is hard work, and they’re to be commended for doing it. And speaking of Bipolar Disorder in children:
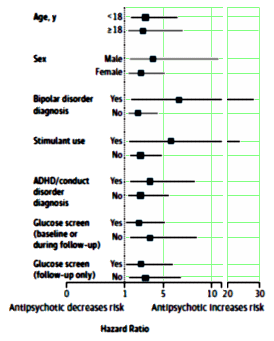
Three points: First, We often read that the pharmaceutical industry has a real burden because it’s so hard to get drugs approved. I would just say that once they get on the market and turn out to be much more toxic that advertised, it’s not very easy to spread that word or get adequate warnings approved either – the black box warning on antidepressants in youth to wit. Look how long it’s taken to get this well-known diabetes issue on the table. Second, the tactic of asking for "more research" to postpone action is a delaying technique until the patent runs out. That is clearly evident in this story of antipsychotics in children. And finally, when I read articles like Dr. Lieberman’s Time to Re-Engage With Pharma? or Dr. Friedman’s A Dry Pipeline for Psychiatric Drugs [where he says we just need to accept more risk and wants to "… entice the drug makers to reinvest in psychiatric drug development"], I wonder what my colleagues are thinking. Actually, I wonder if they’re my colleagues at all, or for that matter, I wonder if they’re even thinking.
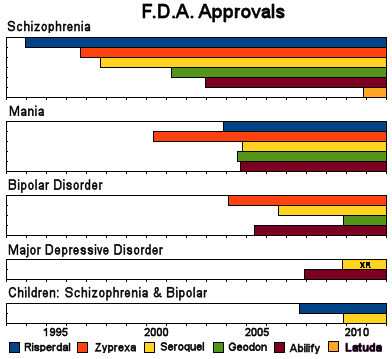 I still wince when people say, "psychiatrists think …" as if we are of one mind and APA President Jeffrey Lieberman or NYT contributor Richard Friedman are speaking for the collective. I want to react and say, "but this author, Mark Olfson, and the authors at Vanderbilt, are psychiatrists; David Healy is a psychiatrist; Ben Goldacre is a psychiatrist too; etc." But I don’t say those things because if I weren’t a psychiatrist, I expect I’d say that same "psychiatrists think …" thing myself. I’ve personally lived through an era when some kind of medication, presently available or as yet undiscovered, became the answer to mental illness. The truth is that medication is only sometimes an answer, or part of an answer, but it never was nor never will be the answer. Sometimes it’s vital, but that’s not very often. We all know that, and I think anybody with good sense has always known that. It’s the getting back to what we all know that’s the current dilemma. We’re twenty years beyond the introduction of the first Atypical Antipsychotic, and the results of this current study were predictable in those very first years. There’s no excuse for that time lag – it was actively created. But the task for the moment continues to be the same, making sure that results like these have a palpable impact on what psychiatrists think…
I still wince when people say, "psychiatrists think …" as if we are of one mind and APA President Jeffrey Lieberman or NYT contributor Richard Friedman are speaking for the collective. I want to react and say, "but this author, Mark Olfson, and the authors at Vanderbilt, are psychiatrists; David Healy is a psychiatrist; Ben Goldacre is a psychiatrist too; etc." But I don’t say those things because if I weren’t a psychiatrist, I expect I’d say that same "psychiatrists think …" thing myself. I’ve personally lived through an era when some kind of medication, presently available or as yet undiscovered, became the answer to mental illness. The truth is that medication is only sometimes an answer, or part of an answer, but it never was nor never will be the answer. Sometimes it’s vital, but that’s not very often. We all know that, and I think anybody with good sense has always known that. It’s the getting back to what we all know that’s the current dilemma. We’re twenty years beyond the introduction of the first Atypical Antipsychotic, and the results of this current study were predictable in those very first years. There’s no excuse for that time lag – it was actively created. But the task for the moment continues to be the same, making sure that results like these have a palpable impact on what psychiatrists think…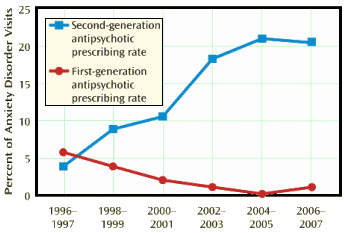
As Dr. Mickey says: “I wonder if they’re even thinking.”
Recently I spoke to a psychiatrist who’s responsible for quality of care or some such in the Psychiatry department of a major US medical center. I mentioned FDA warnings. She scoffed at that — apparently in her world the FDA is perceived as a nervous Nellie whose warnings may be conveniently ignored. (And God knows the FDA has to be strenuously goosed to even produce such warnings.)
What kind of warnings would she and her colleagues take seriously? We already know they don’t stay current with journal articles. Because they can label any patient injury as a psychiatric disorder they’re mostly protected from lawsuits.
What does it take to get clinical psychiatry to pay attention to risks that translate into actual patient harm?
I fear most psychiatrists looking at the link between antipsychotics and diabetes in youth (and everyone else) will dismiss its importance. After all, diabetes is treatable. You could just throw in some metformin with an atypical antipsychotic, problem solved. And the overall health of the individual — well, that’s for the GP to deal with, not a psychiatrist.
Maybe this is nature’s way of depleting the surplus population?
“You could just throw in some metformin with an atypical antipsychotic, problem solved.” Unfortunately (and unsurprisingly) as this VERY concerning JAMA Psychiatry article comes out, so does the framework of an industry/academic NIMH funded paper pushing just this approach: Improving metabolic parameters of antipsychotic child treatment (IMPACT) study: rationale, design, and methods. Child and Adolescent Psychiatry and Mental Health 2013, 7:31. see http://www.capmh.com/content/7/1/31/abstract and full paper PDF here http://www.capmh.com/content/pdf/1753-2000-7-31.pdf Disclosures equally unsurprising – Disclosures – The aripiprazole for this study was donated by Bristol-Myers Squibb. Dr. Sikich has received funding from Bristol-Meyers Squibb for a sponsored clinical trial and donation of medication and patient travel expenses for an investigator initiated study of aripiprazole. Dr. Correll has been a consultant, advisor, lecturer and/or data safety monitor to or has received honoraria from: Actelion, Alexza; AstraZeneca, Biotis, Boehringer-Ingelheim, Bristol-Myers Squibb, Cephalon, Desitin, Eli Lilly, Genentech, Gerson Lehrman Group, GSK, Intracellular Therapies, Lundbeck, Medavante, Medicure, Medscape, Merck, National Institute of Mental Health, Novartis, Ortho-McNeill/Janssen/J&J, Otsuka, Pfizer, ProPhase, Roche, Schering-Plough, Sepracor/Sunovion, Supernus, Takeda, Teva and Vanda. He has received grant support from BMS, Feinstein Institute for Medical Research, Janssen/J&J, National Institute of Mental Health (NIMH), National Alliance for Research in Schizophrenia and Depression (NARSAD), and Otsuka. Dr. Hamer has been a consultant, advisor, or data safety monitor to or has received honoraria from: Abbott, Acadia, Allergan, Alkermex, Alpharma, AstraZeneca, Cenerex, Corcept, Eli Lilly, Endo, Epix, J&J, NeuroPharmaBoost, Novartis, PureTech ventures, Pfizer, Roche, Sanofi-aventis, Schwartz, Solvey, Takeda, Wyeth, and NeurogensX, Inc. Dr. Hamer and/or his spouse own stock in Bristol-Myers Squibb, Amgen, Lilly, genetech, Proctor and Gamble, and Sepracor. Acknowledgement The IMPACT study is funded by NIMH R01 MH080270-01A2.
To follow up on rpurssey’s point, look at the discussion section over at Pharmalot:
http://www.pharmalive.com/antipsychotics-can-triple-the-risk-that-children-develop-diabetes#comments