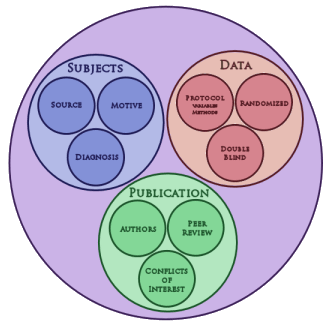
If a Diagnosis is a proxy [stand-in] for my patient, the Subjects in a clinical trial are a collective proxy for the Diagnosis – other people with the same Diagnosis. There are some things I ought to know about those subjects. Where did they come from? What were their motives in being in the trial? How was the Diagnosis made? Here’s another temptation to wander [exploring all these questions], but I’m going to pass for a reason. I figure my interest and the interest of the people doing the study are congruent. Even the marketing department of the most profit seeking pharmaceutical company wants the subjects to have the Diagnosis their drug targets. If they don’t, the study is going to be a bust. So there’s no big disconnect here. PHARMA and I are in sync.
 The next step in the process is gathering the Data – the study itself. And there are some rules, lots of rules, about how a drug trial proceeds. Before starting, there’s a Protocol defining how the study will be done, including: what measurement instruments will be used to collect the Data: what outcome variables will be assessed; and what criteria will define success or failure. The subjects are assigned to placebo and one or more treatment groups by some neutral algorithm. The investigators, the raters, and the subjects are blind to the group assignment. In the process, the Data becomes a proxy for the Subjects, converting the Subjects’ responses into tables and tables of information. This proxy thing really is a lot like those nested Russian dolls. This process is heavily regulated and my patient’s interests are still congruent with the trial’s sponsor’s. How did it come out? Was it efficacious? What were the adverse effects? We’re all at least close to being on the same page before the trial’s blind is broken.
The next step in the process is gathering the Data – the study itself. And there are some rules, lots of rules, about how a drug trial proceeds. Before starting, there’s a Protocol defining how the study will be done, including: what measurement instruments will be used to collect the Data: what outcome variables will be assessed; and what criteria will define success or failure. The subjects are assigned to placebo and one or more treatment groups by some neutral algorithm. The investigators, the raters, and the subjects are blind to the group assignment. In the process, the Data becomes a proxy for the Subjects, converting the Subjects’ responses into tables and tables of information. This proxy thing really is a lot like those nested Russian dolls. This process is heavily regulated and my patient’s interests are still congruent with the trial’s sponsor’s. How did it come out? Was it efficacious? What were the adverse effects? We’re all at least close to being on the same page before the trial’s blind is broken.
In the last several years, I’ve wandered through a lot of these clinical trials, mostly to do with psychiatric drugs. The problems and misbehaviors have become the stuff of legend already, and we’re still close to the starting gate in terms of what needs to be known. It’s obvious that the problematic link in this chain of proxies is in the resulting Publication [see – I made it back around] – the thing that makes the whole thing public. The Publication [journal article] is the proxy for the Data – the end of a long sequence of proxies that started back with my patient and the clinical problem on the table in my office.
Five years ago, I didn’t know that was a problem. I’m a voracious reader, but my area of practice pointed to a different literature. But I don’t think most of my colleagues who weren’t a part of all the sheenanigans knew it either. We had all counted on the integrity of the Journals and the Authors to certify that step in the chain. We had anonymous peer reviewers to go over articles in depth for us. But that has all changed. People have tried lots of fixes in recent years: a war on ghost authorship; demanding to know the funding source; insisting on conflict of interest information. I expect those things have helped some, but the problems remain. They tell us when to be skeptical, but not the real results. And they haven’t solved the problem of the sea of unpublished negative studies. We’ve increasingly looked to independent meta-analyses, but they’re hard to do and hard to fund. In addition, meta-analysis lags way behind clinical usage. And meta-analyses are prey to the same kind of problems as the articles themselves [eg a biased meta-analysis of biased analyses – plenty of examples].
A year ago, I was obsessing about how to plug the holes, inventing things to do that would make the Publications what they should be – accurate proxies for the Data. I had something of a knot in my stomach because everything I thought of meant heavily regulating our Academic Journals like the clinical trial process is regulated, and that seemed absurd, like something important would be lost. About that time, GSK was finally induced to put their data for Paxil Study 329 on-line. My analysis was crude, several Excel Spreadsheets and more time than I’d like to admit copying numbers into them by hand. But, in spite of being a crude shot with few tools, it was easy to see in a solid way that everything we thought about that study was true – it was a negative study. The Adverse Events weren’t right either. And the problem wasn’t in the Protocol, or the conduct of the trial, in the Data. It was in the Publication – the thing that made it public. That publication was not a proxy for the Data.
I’m not sure if others are having this problem, but after appendix: a primer if you haven’t seen it:
I just see a blank.
My, bad I was viewing on something that didn’t bring up the TED video. Can see it now.
Everyone — sign this petition calling on Richard Gonzalez, the CEO of AbbVie to drop his legal action against the EMA’s policy of open access to clinical trial data. The petition is here
http://chn.ge/13clTyF
or in Spanish, French, German, Han and Hindi on Rxisk here
http://wp.me/p2FaK9-Rf
[the above from Rxisk.org]
Please pass this along.
I also just see a blank after the Appendix line. Which Ted Talk is it?
PsychPractice,
Here is the link to Ben Goldacre’s “Battling Bad Science”:
http://www.ted.com/talks/ben_goldacre_battling_bad_science.html
Anyone who has ever been at the mercy of publicly paid mental health professionals has probably seen the effects of working with professionals who don’t have access to the latest of anything. The Rosenham study is behind a paywall. I’m of the opinion that it;s also important for the general public to have access to studies and papers that address the reasoning behind decisions and perspectives in the field of psychiatry.
And the current proxy for “Anonymized Data” is?
http://www.ihealthbeat.org/articles/2013/7/22/states-mull-policies-on-collection-sale-of-deidentified-patient-data
http://www.businessweek.com/news/2013-06-05/states-hospital-data-for-sale-leaves-veteran-s-privacy-at-risk
On a lighter note I found this abstractocity at Retraction Watch:
http://retractionwatch.wordpress.com/2013/09/23/a-serbian-sokal-authors-spoof-pub-with-ron-jeremy-and-michael-jackson-references/#comments