 |
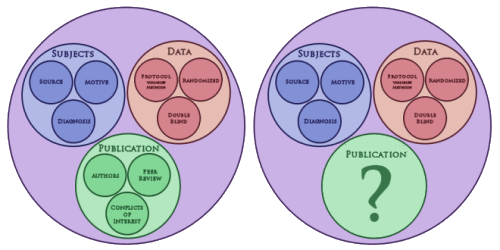
| A few days ago, I was talking about a Venn diagram [in proxies…] I’d sketched on the back of an envelope [left above]. A more accurate version of what it really looked like originally is on the right. What I was trying to get at is that in the Clinical Trial process, the motives of the pharmaceutical company and the motives of doctors and patients may differ somewhat, but the overall result is the same. The drug company moguls would be pleased as punch if the RCT blind was broken and revealed a new medication that was effective and non-toxic. So their motives and ours are similar in the top two bubbles, gathering and testing a subject cohort that has the right condition and is powered to show the desired result. Where things go haywire is when the study comes back with results that are tempting, but lacking robustness, safety, or both. So close and yet so far! That’s when the trouble starts. That’s where the temptation to "latch on to the affirmative, eliminate the negative, … and don’t mess with mister in-between" too often comes into the picture. It’s in what happens in the green bubble, the space between breaking the blind and the published paper. That’s where the deceit lives and has flourished in epidemic proportions behind closed doors. |
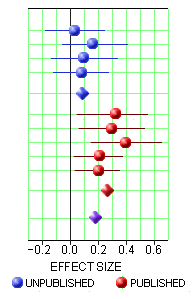
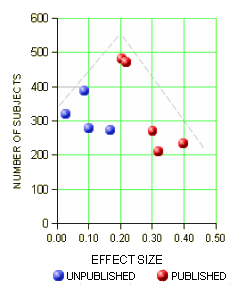
One way to deal with a negative or not quite positive study is to simply ignore it, not publish it. I hadn’t been aware of what a powerful tool that could be. With the FDA, you have to include it in the safety evaluation, but you can leave it out altogether in the efficacy submission. The FDA requirement is two positives. If you’ve got a bunch of negative trials, no matter. So if you get it through the FDA, the clinicians have no clue about the raft of failed attempts until Cochrane or someone else does a meta-analysis late in the patent life of the drug [if they can find out about the negative studies!]. This is where the AllTrials campaign shines. By insisting that something be published from every trial, we’ll know about all the duds along the way up front. I gave an example recently from the Agomelatine studies [a breath of fresh air…]:
 |
| Why bother to change the green bubble? The first time around, I populated it with the things that ought to protect us from the deceitful papers we’ve been inundated with, particularly in industry sponsored trials, particularly in psychiatry. But that’s obviously not enough, because they kept on coming. Recently, I’ve been looking back over some of those papers thinking about how to keep them out of the literature, or at least keep them out in the flavor they now come in. I guess I was wondering if there might be some way of adding regulations like we have with the other aspects of the process. But the things that are deceitful are subtle and have an almost infinite variety. And I’m not sure I’d want to routinize publications anyway. It’s stifling. So what to do? Thus, the big question mark… |
Almost anything more than what we have would do something. If the data reports required by clinicaltrials.gov were really required, that would’ve been enough to stop a lot of the corruption. The study reports proposed in AllTrials would do the same thing. Those things work because the mechanisms of deceit are in what’s omitted or in the selective computation and presentation of what is shown. So why isn’t everyone dancing in the streets from the progress made already? Why does Dr. Healy keep writing these pesky doomsday articles like Neal Parker Avoiding Adverse Events? Why does Johanna leave us comments like this one? For that matter, why do I post something like the devil’s still in the details… every week or so? Even the nice guys like Ben Goldacre and Fiona Godlee go to parliamentary committees and can get a bit whiny on this topic [goldacre and godlee…, a sticky wicket…]? The answer is pretty simple. Anything [I mean any- and every-thing] that is prepared by the manufacturer is subject to the same manipulation as these ghost-written jury-rigged articles, no matter what the rules. That’s why. The stakes for them are too high. The temptations are too great.
I’ve been looking over some of the older studies, ones I looked at back when I first developed this hobby of reading the psychiatric clinical trial literature several years ago. I’m amazed at how easy it is now to see the subtle ways they’ve been written to lean the truth, or omit it, even in some of the non-industry sponsored articles. I’m certainly no ace at spotting all the twists and turns possible, but I’m so much better at it now than I was just five years ago. And back then, I would’ve thought I should’ve been better than most, having been a research type in a former career. I’m still awed at some of the friends I’ve made along the way who have actually been involved in pharmaceutical research and clinical trials. They really do have "the gift" – a sixth sense. They smell deceit. I expect I’ll go through some of those older trials here as examples in the not distant future.
But for the moment, the point is this. No matter how the AllTrials campaign or the EMA data transparency program comes out, there is a bottom line. There has to be a provision that allows access to the data as it came off the press at the last point when the goals of the pharmaceutical company and the needs of the patient who is going to be offered the medication are the same. And that moment is when the raw data as it stands when the blind is broken is revealed. As long as that information is in a safe place and there’s access for checking [for no other reason than it needs checking], we’ve done all we can do. Any program that falls short of that is a loophole awaiting exploitation. So everything we are doing right now will help, but without that essential piece, the job has not been completed and the question mark remains…

I think this should extend to all papers, not just trial data. The Rosenham Experiment is behind a paywall. I think everything that comprises past and current views in psychiatry needs to be made public within a year of publication. The public has a right know, imo, and psychiatrists should be held accountable to the degree that they must spell out their views before charging anyone for a visit.
The fact that it’s nearly impossible for a person to have a diagnosis removed is evidence enough that the doctor/patient relationship in psychiatry gives psychiatrists too much power with too little checks and balances and therefore lends itself to the abuse of people that are supposed to be being helped.
I think the FDA should be forced to make all data submitted public. We pay tax dollars to them to regulate the health industry, they cannot justify doing it in near complete secrecy from the scientific community. How absurd is that.
– and complete secrecy from the public!
The drug labeling tells us what the FDA wants us to hear, not whether they they made the right decision. That’s for the scientific community to decide. The approval of antipsychotics in children is a good example. The FDA said they did the approval because off-label use was already endemic and they wanted the industry to do trials on children. We were just suposed to trust the drug labeling information that told us 2 out of 5 trials is negative over placebo? Outragious.
Then we see stuff like this published:
“Twenty-four months of antipsychotic treatment in children and adolescents with first psychotic episode: discontinuation and tolerability.”
http://www.ncbi.nlm.nih.gov/pubmed/23771198
This data suggested the approval was a mistake.
but the FDA keeps everything locked away. Saftey and efficacy data is a trade secret. Maybe that’s how the FDA covers it’s ass.
Wiley,
The point you bring up is certainly valid – and an impossible dilemma. If a patient uses insurance, a diagnosis is a requirement. My understanding is that any mental health diagnosis then can become a liability. The only way around that is to refuse to fill out insurance forms, which means no reimbursement. I think this liability-by-diagnosis extends to all of medicine as well, though perhaps without the stigma of psychiatric diagnoses. I think that the current reforms were designed to eliminate the business of pre-existing conditions, but I doubt that they do much for the stigma part. This is a huge problem. Even in a charity clinic, the only thing I write is something like “patient seen” and medications, if any. So instead of the chart being a communication, it’s a potential liability. I don’t know what the solution is to this. I resent the system too. I expect most do, because it’s a double bind of the first magnitude.