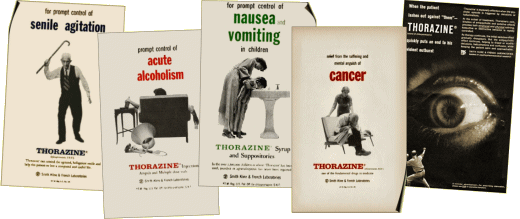
[SmithKlineFrench in former days]
 The corporate history of GlaxoSmithKline [GSK] starts with a Drug Store in Philadelphia [1830] and a Trading Company in New Zealand [1873], a history sketched through the many mergers on their website. But the parts that are of current interest were the acquisition of SmithKlineBeecham by GlaxoWelcome in 2000 and the coming of Andrew Witty as CEO in 2008. Witty has been with Glaxo since 1985, straight from university at age 21 – rising through the ranks.
The corporate history of GlaxoSmithKline [GSK] starts with a Drug Store in Philadelphia [1830] and a Trading Company in New Zealand [1873], a history sketched through the many mergers on their website. But the parts that are of current interest were the acquisition of SmithKlineBeecham by GlaxoWelcome in 2000 and the coming of Andrew Witty as CEO in 2008. Witty has been with Glaxo since 1985, straight from university at age 21 – rising through the ranks.
 The story would be incomplete without yet another character, Eliot Spitzer, then Attorney General of New York who settled a lawsuit against GSK in 2004 over false claims about using their antidepressant Paxil in children and adolescents [Study 329]. The financial part of the settlement was insignificant, but this part wasn’t at all trivial:
The story would be incomplete without yet another character, Eliot Spitzer, then Attorney General of New York who settled a lawsuit against GSK in 2004 over false claims about using their antidepressant Paxil in children and adolescents [Study 329]. The financial part of the settlement was insignificant, but this part wasn’t at all trivial:In addition to the monetary portion of the settlement, GSK has also agreed to publicly disclose all of its clinical drug trials about the safety of an antidepressant for children. The company will put summaries of all of its studies since December 2000 in a clinical trial registry on its Web site…
Forbesby Matthew Herper10/12/2012In an unprecedented move that could signal dramatic changes in the drug industry, GlaxoSmithKline is promising to make detailed data from its clinical trials available to independent researchers so that scientists can draw their own conclusions about the safety and effectiveness of its new drugs. The change, which has yet to be implemented, is being announced at a speech in London at the Wellcome Trust, where Glaxo chief executive Andrew Witty is also detailing how the British drug giant has made its chemical libraries available to researchers working on drugs against tuberculosis and malaria. It could be a dramatic change for a company that has been dogged by scandal over lack of disclosure.
“Because of our unique role, we recognize that society holds us to higher standards than for other industries,” Witty says in his prepared remarks, which may change. “This is how it should be. Over the last four or so years we at GSK have been working hard to be more open and transparent. As I have shown these new approaches are helping to provide new solutions for serious global health issues. They will also help build society’s trust.”Right now, Glaxo publishes results from its clinical trials on its own Web site and on another site run by the National Institutes of Health, and it says it tries to publish scientific papers on every study in research journals. But doctors outside the company don’t have access to vast databases of how each patient in a clinical trial did. What Glaxo is promising to do is to create a process through which researchers can request this raw data and use it to do new analyses…
Witty, who became Glaxo’s chief executive in 2008, has tried to distance the company from the Avandia controversy, focusing attention on the company’s efforts in the developing world, including research to create the first vaccine for malaria and now, announced today, new initiatives against tuberculosis and other emerging diseases. In Glaxo’s press release announcing the settlement with the feds, he said that the Avandia and Paxil controversies “originate in a different era for the company” and expressed his “regret” and said that his company had learned from its mistakes.
What Witty is saying he will do now goes way beyond anything even Eliot Spitzer asked for. Glaxo would put in place a system by which independent researchers could request the data about what happened to individual patients in its clinical trials, and would be granted access if an independent group of experts thought the idea had scientific merit.
“We think that it’s the right thing to do for patients, we think it’s the right thing to do for understanding our medicines,” says Patrick Vallance, the senior vice president in charge of drug research at Glaxo. “I think if you volunteered to be in a clinical trial, your legitimate expectation is that your data will be used to insure that future generations of patients get the maximal advantage.” Vallance denied that the initiative was a response to past scandals. “It’s absolutely what I’ve believed for a long time. It’s what Andrew believed. It’s what many people in the company believed”…
Access to Patient-Level Data from GlaxoSmithKline Clinical Trialsby Perry Nisen, M.D., Ph.D., and Frank Rockhold, Ph.D.New England Journal of Medicine. 2013 369:475-78.
What information will be made available?GlaxoSmithKline will provide the raw data set [the data collected for each patient in the clinical study] and the analysis-ready data set [the data set analyzed by GlaxoSmithKline and provided to regulatory authorities]…
What information will investigators be required to submit?It is important that the analyses proposed by investigators petitioning to access a data set have scientific credibility. We believe that there are public health risks if the proposed analyses are not scientifically robust and give rise to erroneous concerns about safety or false hopes of a potential benefit for patients. Therefore, in accordance with the expectations of usual good scientific practice, investigators will be required to submit a brief research proposal with the use of an online form [Section 2 in the Supplementary Appendix], describing their analysis and publication plans, their management of potential conflicts of interest, and the qualifications and experience of their search team [which should include a statistician]…
How will research proposals be reviewed?After they have been processed to ensure that the submitted information is complete, proposals will be reviewed by an independent review panel. The panel will initially comprise external experts appointed by GlaxoSmithKline. The independent review panel will accept or reject proposals on the basis of their scientific rationale and relevance to medical science or patient care. The panel will also consider the qualifications of the investigators and the management of potential conflicts of interest. To make the decision-making process fully transparent, we provide a list of the members of the independent review panel and their charter on the website…What are the conditions on which access will be provided?Investigators will be responsible for obtaining any other approval that may be required for their research [e.g., from ethics committees, institutional review boards, relevant research institutions, or funding bodies]. They will also be required to sign a data-sharing agreement that commits them to use the data only for the research purpose described in the accepted research proposal…
[beginning at 1:37] “Well what we’ve done – well sequentially we’ve been increasing the level of data transparency around the data we generate on clinical trials. So we’ve been publishing clinical trials summaries for a while. We’ve now committed the publish all of our clinical trial reports that are detailed reports of those trials, and also the patient level data. Now nobody else in the world is committed to doing that…[beginning at 2:02] “And we’re not simply going to publish data on trials still to come, but we’re going to go back and we’re going to publish all the data for all the trials that have been done since the company was formed… since 2000.”[beginning at 2:33] “We’re doing it because we think it’s in the interest of patient safety, first of all, so that it gives as many people as want to look at the data a chance to make sure that absolutely the right conclusions have been drawn. If we’ve missed something, we want to hear about it…”
Do I have the credentials they might find suitable? Would my primitive statistical expertise stand their test? Am I capable of tallying up the adverse events appropriately? And what’s my research proposal? All I want is to make sure that absolutely the right conclusions have been drawn. In fact, I did that with their Study 329 data when it finally showed up on their website last year [some eleven years after they published the article, some seven years after they were court ordered to post the data publicly – see the lesson of Study 329: an unfinished symphony…]. The data was in a text format so I had to copy the values by hand to a spread sheet. And as you know, that study was negative every which way but Sunday, unlike the published article’s conclusions.
It is important that the analyses proposed by investigators petitioning to access a data set have scientific credibility. We believe that there are public health risks if the proposed analyses are not scientifically robust and give rise to erroneous concerns about safety or false hopes of a potential benefit for patients. Therefore, in accordance with the expectations of usual good scientific practice, investigators will be required to submit a brief research proposal with the use of an online form, describing their analysis and publication plans, their management of potential conflicts of interest, and the qualifications and experience of their search team [which should include a statistician].
This blog and many others are full of similar examples – unpublished studies, studies with biased design, statistical manipulations, omissions, creative graphs, etc. There’s even a growing field in medicine of people who spend their time using creative but indirect methods to find all the games being played to distort the Clinical Trial results. The record speaks for itself, and GlaxoSmithKline is prominently represented. I really like and respect the work Iain Chalmers and Ben Goldacre have done. I fully support AllTrials. I own Bad Science and two versions of Bad Pharma. I even think I might like Andrew Witty if he lived on my street. But, if he’s going to say he has a policy that gives as many people as want to look at the data a chance to make sure that absolutely the right conclusions have been drawn, he’s going to have to make his company’s actual policy match the words.
A 50-something psychiatrist told me that the people running SmithKlineBeecham had been psychopaths and Glaxo bought their messes.
But since then, GSK has hardly distinguished itself as a beacon of trustworthiness.
http://pharmagossip.blogspot.com/2013/10/1boringoldman-hits-it-out-of-ballpark.html
True
Here’s a little twist. The descendent of the family who owned the Trading Company in New Zealand is now our Prime Minister’s Chief Science Advisor and an avid supporter of drug treatment for suicidal kids.