by Steven J. Garlow, Becky Kinkead, Michael E. Thase , Lewis L. Judd, A. John Rush, Kimberly A. Yonkers , David J. Kupfer, Ellen Frank, Pamela J. Schettler, and Mark Hyman RapaportJournal of Psychiatric Research 2013 47:1199-1203.
Objective: Some reports suggest an increase in suicide ideations and behaviors in patients treated with antidepressants. This is an analysis of the impact of fluoxetine on suicide ideations in outpatients with minor depressive disorder.Methods: Research subjects were adult outpatients with minor depressive disorder [N = 162], who received fluoxetine or placebo in a prospective, 12-week, double-blind randomized trial. The research participants were evaluated weekly with standard rating scales that included four suicide-related items:• item 3 of the Hamilton Rating Scale for Depression [HRSD],• item 18 of Inventory of Depressive Symptomatology [IDS-C], and• items 15 and 59 of the Hopkins Symptom Checklist [SCL-90].Clinically significant intensification of suicide ideation was defined as an increase of 2 points on any of these items.Results: Overall 60/162 subjects [37%] had an increase of 1 point during treatment and 17/162 [10.5%] of 2 points on at least one suicide item, with 12/81 [14.8%] placebo and 5/81 [6.2%] fluoxetine-treated subjects having a 2 point gain. Of the study participants with baseline suicide ideation, 9/22 [40.9%] placebo and 3/24 [12.5%] fluoxetine treated had 2 point increase [p = 0.04]. Survival analysis revealed that subjects on placebo were significantly more likely [p = 0.050] to experience a 2 point increase on one or more item, a difference that emerged early and continued throughout the 12-week trial.Conclusions: Compared to placebo, fluoxetine was not associated with a clinically significant increase in suicide ideation among adults with minor depressive disorder during 12 weeks of treatment.
The authors would like to thank John Plewes MD for his enthusiastic encouragement and support. This study was supported in part by NIMH grants MH-30914, MH-49671, MH-30915, MH-53799, and MH-01648-01; the Roher Fund of the University of California, San Diego; and an unrestricted investigator-initiated contract from Eli Lilly & Co.
"There are certain limitations to this study that need to be considered when evaluating the results. This is a secondary analysis of an efficacy trial that was not specifically designed or powered to address the potential relationship between fluoxetine treatment and suicide ideation. The total number of subjects in the subgroups who experienced a worsening of suicide ideation is small so the study may be under-powered to fully evaluate this effect. Another potential limitation is the focus on rating scale-based assessment of suicide risk, with no other outcome measures such as adverse event reports, as has been employed in other analyses of this question… Patients with HRSD item 3 score or IDS-C item 18 above were excluded as were those considered at high suicide risk by the investigators during screening. Therefore, the study sample may have been depleted of those most at risk to experience antidepressant-induced treatment emergent suicide ideations and behaviors and so underestimate this outcome. A final consideration is that this trial included adults with minor depressive disorder, with mean age of 43.5 years so the results cannot be extrapolated to adolescents and young adults…"
by Mark Hyman Rapaport, M.D.; Lewis L. Judd, M.D.; Pamela J. Schettler, Ph.D.; Kimberly Ann Yonkers, M.D.; Michael E. Thase, M.D.; David J. Kupfer, M.D.; Ellen Frank, Ph.D.; John M. Plewes, M.D.; Gary D. Tollefson, M.D., Ph.D.; and A. John Rush, M.D.American Journal of Psychiatry 2002 159:637-643
[full text on-line]
OBJECTIVE: The authors provide a detailed clinical description of minor depression: its symptoms, level of disability, stability, and relationship to patient and family history of major depressive disorder.METHOD: Rigorous criteria for minor depression, including functional disability, were used to identify 226 individuals for a three-phase treatment study. This report presents data obtained on that study group during the first study phase, a 4-week placebo lead-in period.RESULTS: One hundred sixty-two subjects [72% of the initial study group] remained in the study for 4 weeks and continued to meet criteria for minor depression. Minor depression in these subjects was primarily characterized by mood and cognitive symptoms, not the classical neurovegetative signs and symptoms of depression. Approximately one-third of the subjects with minor depression had a past history of major depressive disorder, and nearly half had a family history of unipolar depressive disorder; however, neither factor affected the severity or quality of minor depressive symptoms.CONCLUSIONS: These data suggest that:[1] minor depression is not evanescent;[2] minor depression is characterized by mood and cognitive symptoms rather than neurovegetative symptoms;[3] minor depression may occur either independently of a lifetime history of major depressive disorder or as a stage of illness in the course of recurrent unipolar depressive disorder; and[4] depressive disorders should be conceptualized as a continuum of severity.
From the Psychopharmacology Research Program, Department of Psychiatry, University of California, San Diego, School of Medicine; the Psychiatric Service, VA Healthcare System, San Diego; Eli Lilly and Co., Indianapolis; Western Psychiatric Institute and Clinic, Department of Psychiatry, University of Pittsburgh; and the Department of Psychiatry, University of Texas, Southwestern Medical Center, Dallas… Supported by an unrestricted research grant from Eli Lilly and Company; by the National Alliance for Research on Schizophrenia and Depression [Dr. Rapaport]; and by NIMH grants MH-30914, MH-49746, MH-80001 [Dr. Rapaport], MH-01908 [Dr. Yonkers], MH-30915 [Dr. Kupfer], MH-49115 and MH-30915 [Dr. Frank], and MH-53799 [Dr. Rush]. Dr. Schettler is a statistical consultant to Eli Lilly and Company.
by Lewis L. Judd, M.D.; Mark Hyman Rapaport, M.D.; Kimberly A. Yonkers, M.D.; A. John Rush, M.D.; Ellen Frank, Ph.D.; Michael E. Thase, M.D.; David J. Kupfer, M.D.; John M. Plewes, M.D.; Pamela J. Schettler, Ph.D.; and Gary Tollefson, M.D., Ph.D.American Journal of Psychiatry 2004 161:1864-1871.
[full text on-line]
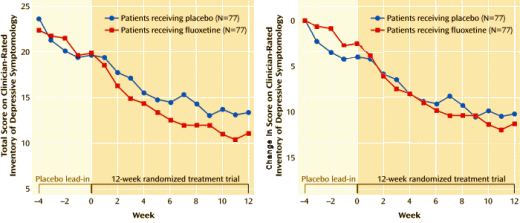
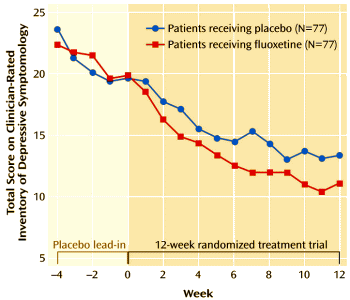
OBJECTIVE: Minor depressive disorder is both common and associated with significant psychosocial impairment. This study examined antidepressant treatment efficacy in a large group of patients with minor depressive disorder.METHOD: One hundred sixty-two patients with minor depressive disorder were randomly assigned to receive fluoxetine or placebo in a 12-week, double-blind study; 73% [59 of 81] of the patients in each treatment group completed the study. Patients were evaluated weekly with standard depression rating instruments and measures of psychosocial impairment. Hypotheses were tested by last-observation-carried-forward analysis of variance [ANOVA] and confirmed by mixed [random-effects] regression analysis.RESULTS: At baseline, minor depressive disorder patients were mildly to moderately depressed, with a corresponding degree of functional impairment. Over 12 weeks of treatment, both ANOVA and mixed regression showed fluoxetine to be superior to placebo as indicated by significantly greater improvement of fluoxetine-treated patients in scores on the 30-item clinician-rated Inventory of Depressive Symptomatology, the 17-item and 21-item Hamilton Depression Rating Scale, the Beck Depression Inventory, and the Clinical Global Impression severity scale. Improvement in Global Assessment of Functioning Scale score was significantly greater for the fluoxetine group in mixed regression analysis only. Patients in both treatment groups reported a similar number and severity of adverse events during the 12-week treatment period.CONCLUSIONS: Clinicians frequently encounter minor depressive disorder either as a prodromal or residual phase of illness in major depressive disorder or as de novo minor depressive disorder episodes. Fluoxetine is significantly superior to placebo in reducing minor depressive disorder symptoms within a 12-week period. Improvement in psychosocial function with fluoxetine may take longer than 12 weeks.
… and Eli Lilly & Co., Indianapolis [Drs. Plewes, Schettler, and Tollefson]… Supported in part by NIMH grants MH-30914, MH-49671, MH-30915, MH-53799, and MH-01648-01; the Roher Fund of the University of California, San Diego; and an unrestricted investigator-initiated contract from Eli Lilly & Co.
The timing of these articles is interesting. Prozac was FDA Approved in December 1987, and I suspect this study was a move to extend the indications from MDD to the ubiquitous miD, even weighing in on the side of inclusion of miD in the DSM-IV. Failing at that, the trial was tabled for a long time, missing in action. So why exhume it in 2002 and 2004?. I speculated on two reasons: the coming of the DSM-5 and the eminent release of Lilly’s Cymbalta®. But maybe the authors just wanted another publication. It was unlikely related to Prozac® as that drug went off-patent in August 2001. By my reckoning, this study was a classic experimercial – a clinical trial done for marketing purposes rather than driven by any scientific goal – aimed at extending the target population for Prozac®. One of the many that litter our literature.
Now it’s twenty years later and this Clinical Trial has been revived in a form disconnected from its origins – one in a long line of articles that appear at regular intervals contesting the FDA Black Box Warning. A lot of them are some kind of retrospective like this one or the articles of Robert Gibbons extensively reviewed here [an anatomy of a deceit…]. Some look at suicide statistics [NBER study] or drug sales [Impact of Publicity Concerning Pediatric Suicidality Data on Physician Practice Patterns in the United States]. Some even review media articles [News coverage of FDA warnings on pediatric antidepressant use and suicidality]. Like this particular offering, they are often freebies – analyzing some existing dataset rather than doing an expensive new study. They often use some surrogate index or ancient data intended for some other purpose. Lilly has been involved in a lot of them.
While one might wonder why these articles keep coming, particularly from the Lilly/Prozac® camp [since Prozac® has been off-patent for well over a decade and Cymbalta® goes off-patent in a month or so]. But the issue of suicidality associated with the antidepressants has taken on a meaning bigger than the basic question itself [which is already pretty big]. It has come to be a symbol for the concept that mental illness is biological and its treatment is exclusively with medications – an enterprise worth billions to the pharmaceutical industry and defines the role of many of the current generation of psychiatrists.
Whoever it was who added the term experimercial to our lexicon deserves our thanks.
Ivan,
Agreed. It was added by Dr. Bernard Carroll and has, at least on this blog, become the metaphor for the modern Psychiatric literature – there are so many of them:
see A Field Guide for Science Writers
I’ve been reading anything I can get my hands on about the possible link between PTSD in combat veterans and psychosis.* It’s frustrating to read these reports that are attempting to find veterans with PTSD who have had one or more psychotic episodes. Most of these talk about the likelihood that some vets have been misdiagnosed with a mental disorder, while talking about those with and without a comorbid psychotic disorder as if they knew how to make that distinction already..
Then they talk about how they weeded out veterans with a comorbid psychosis for the purposes of separating the possible PTSD-Ps from those who are assumed to have comorbid psychotic disorders— because they’ve been diagnosed with them, possibly also over the assumption that the vets with PTSD and psychosis (stated that they had) mental illness in their family so the vets has, by that logic, a biologically determined psychotic disorder..
So, ipso facto, they have a comorbid psychotic disorder, and the researchers ignore the obvious circularity here. What if the ones with “previous psychotic disorders” were misdiagnosed? I can see why this would be a wise exercise for the purpose of a study, but I can’t see why they wouldn’t acknowledge that those who were diagnosed with psychotic disorders might have been misdiagnosed.
It’s like once they hang a label on you, that’s what you are. Until researchers give you their seal of recognition, there’s no reason to question a prior diagnosis, and if you admit to there being “mental illness” in your family, then that’s evidence enough that you have a comorbid psychotic disorder. No reason to even wonder if any of those family members might also have been misdiagnosed, because evidently misdiagnoses can only be suspected when it’s the subject of an attempt to make another label.
When dealing with vets or immigrant who had experienced extreme violence and were suffering from PTSD and one or more psychotic episodes that were dominated by the content of their trauma, why is it so important to default to “comorbidity” with any of them? Do most psychiatric researchers and clinicians actually have experience with violence of the nature of these people their studying? What makes them think they have any qualification, whatsover to determine whether or not a person’s reaction to such violence is biologically or not? Do they think they know how they would respond to the same kinds of events? Where do they get off? What is it about them that makes them think they’re so blessedly cognizant of the effects of extraordinary trauma?
It seems that once a label is given, researchers and clinicians wash their hands of the people they’re supposed to be helping. I don’t know what to call this professional lack of self-awareness joined with a blaring lack of scientific and social curiosity combines with the ubiquitous problem of corruption in research; but as you say, Mickey, “It’s time for these wars of ideology to be put to rest and for a renewed focus on the care of the sick…“. Until this bio-bio-bio psychiatry and experimercial approach to research gets a kick in the teeth that hurts enough to break the spell, this field will be the kind of parasite that kills the hosts.
* and immigrants who have been politically persecuted and tortured in their native country
Dear Dr Nardo
I am a regular reader of Boring Old Man and so feel quite honored that my paper is the first you chose to highlight on the return from your vacation. I would offer a couple of comments on your critique. The first is that before you make assumptions and possible aspersions about someone
“who remain committed to these exclusively biological·neuroscience·psychopharmacologic models ”
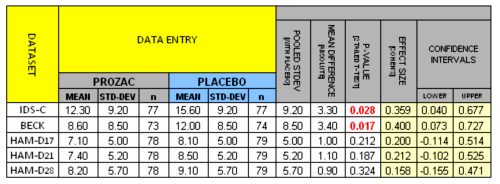
you might want to take the time to get to know something about them, or even better get to know them. Next time you are at Emory lecturing in the psychoanalytic program (I hear your lectures are quite good) ask some of your students about me, or better yet stop by my office and chat, its about 150 feet from Tuft house front door. The actual point of the article, which I reiterated in my letter of response to Stone is on the occurrence of suicide related behaviors in depressed people versus the availability of any treatment. The relationship between antidepressants and suicide related behaviors is rare compared to the emergence of suicide related behaviors in patient who are not treated. In fact in the Stone meta analysis in the limitations section the following statement appears
“Another potential problem in this study is the sparseness
of the data. Many trials had only one event or no
events at all.”
The number needed to harm in the Stone meta analysis for all adults for the next suicide behavior related event was 1000, and 588 in the pediatric, adolescent, young adult group, whereas in our study the NNH for placebo exposure for worsening of suicide ideation was 3.2. This helps contextualize the likelihood of seeing such events on a scale and in patients consistent with manny practitioners in many common practice settings. Practitioners need to be aware of the rare event but need to be equally aware of the common event. Would the minor depression patients in our study have benefited from some non pharmacological intervention such as CBT or IPT?? Possibly, but that would be another study for another day…..