Yesterday, the American Academy of Child and Adolescent Psychiatry wrapped up its 60th Annual Meeting in Orlando Florida. As you might recall, they published Efficacy of Paroxetine in the Treatment of Adolescent Major Depression: A Randomized, Controlled Trial in their journal in 2001, concluding that "Paroxetine is generally well tolerated and effective for major depression in adolescents." I understand that at their meeting last week, Paxil Study 329 was not open for discussion, just like last year when I filed a formal request for retraction. Appeals to the Authors, their sponsoring Universities, the editor of the Journal of the American Academy of Child and Adolescent Psychiatry, the Academy’s leadership and Ethics Committee, the NIH Office of Research Integrity, and GlaxoSmithKline have all been met in this same way. And yet Paxil Study 329 has been central in any number of successful suits against GSK, including last year’s record $3 B settlement and has by now a rich literature of its own.
Pharmalotby Ed Silverman10/28/2013For the past year, GlaxoSmithKline has vowed to usher in a new era of transparency by creating a system to disclose detailed clinical trial data, a widely publicized move that has been hailed by many critics of the pharmaceutical industry who have accused drugmakers of deliberately concealing vital information that should be accessible to others in order to confirm safety and effectiveness. Now, though, a group of researchers is putting the drugmaker to the test by requesting detailed data for an infamous study of its Paxil antidepressant, but are squabbling with the drugmaker over information being sought. In the process, the dispute is raising questions about whether Glaxo complied with a 2004 consent order with the New York State Attorney General to publicly disclose the Paxil trial data.
At issue is data for Study 329. Glaxo participated in preparing, publishing and distributing what US authorities called a "misleading medical journal article" because the results reported that a Paxil clinical trial demonstrated efficacy in treating depression in patients under age 18, when the study actually failed to make the case. The trial missed its endpoints and also figured in a ghostwriting controversy [here is the study]. For more than a decade, the study has haunted Glaxo after it became known that suicide risks in what was one of the best-selling antidepressants had been minimized. The episode, which led Glaxo to the 2004 consent order, factored into the $3 billion settlement with the US Department of Justice last year for illegal promotion and pricing activities [more here]. For its part, Glaxo maintains it has fully complied with the order (more on that later).
However, a loose-knit group of researchers led by Jon Jureidini, a child psychiatrist at the Women’s and Children’s Hospital in Adelaide, Australia, and a professor in psychiatry and pediatrics at the University of Adelaide, is haggling with Glaxo over certain data referred to in the consent order and they maintain the drugmaker has balked at their request and raised questions about its commitment to releasing data. The tussle underscores the ongoing tension over the extent to which certain data – notably, anonymous patient-level data – can or should be released. Last week, a group that includes academics and consultants, some of whom are affiliated with the Multi-Regional Clinical Trials Center at Harvard University, suggested an independent panel should be created [see this]. And a recent essay in The Lancet, Glaxo R&D chief Patrick Vallance and Iain Chalmers, one of the founders of the Cochrane Collaboration, argue that open access to this detailed data poses many risks…
-
The first is obvious – to correct the academic literature. This article shouldn’t be there. If they won’t retract it or correct it, then the only choice is to add a corrected version of the report on the clinical trial.
-
Second, to make the statement to future sponsors, authors, academic institutions, and journal editors that if they engage in this kind of scientific misbehavior, whatever their motive, they should be prepared for a public statement of what they did as a potential consequence.
The later part of the Pharmalot post is about the RIAT team’s request for the full patient-level data from Paxil Study 329, particularly from the Adverse Effects – reporting that is omitted in the online data [Appendix H]. Ed tells that story accurately and I’ll defer to his version. But there’s one piece to add to his report:
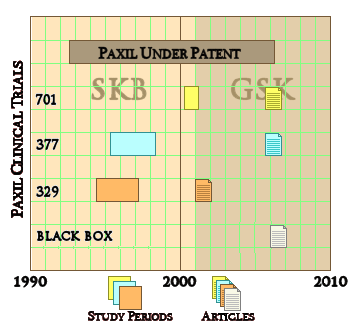
For instance, page 7 of the order says that, beginning in February 2005, Glaxo must provide public access to the clinical study reports of Paxil as a treatment for major depressive disorder in the pediatric population for a 10-year period [here is the consent order]. An attached document defines clinical study report as including all data. And data is defined as all the results and outcome measurements obtained from a clinical study [here is the document].
by Apter A, Lipschitz A, Fong R, Carpenter DJ, Krulewicz S, Davies JT, Wilkinson C, Perera P, and Metz A.Journal of Child and Adolescent Psychopharmacology. 2006 16[1-2]:77-90.CONCLUSIONS: Adolescents treated with paroxetine showed an increased risk of suicide-related events. Suicidality rating scales did not show this risk difference. The presence of uncontrolled suicide risk factors, the relatively low incidence of these events, and their predominance in adolescents with MDD make it difficult to identify a single cause for suicidality in these pediatric patients.

You’re a white knight in the battle for patient safety and accountability for drug makers! Thank you.