-
there’s a long history of erroneous attempts at solving what seems the impossible dilemma when entrepreneurialism collides with rational health care and medical ethics, and the territory between scorched earth and sleeping with the enemy remains to be defined.
-
Why have so many attempts at reform met with such regular failure?
-
…no matter what we do, it has to include both the resources and the mandate for oversight – indefinite, ongoing oversight. No more honor system and grand rhetoric. There’s just not enough honor in the marketplace to get the job done.
-
Companies don’t commit fraud – people do. It’s part of that "cost of doing business" argument that’s so regularly mentioned that it’s become a figure of speech. This is the door we haven’t opened that can’t continue to stay closed.
-
…there’s plenty of out-right crime accompanying the distortions in clinical trial reporting, libraries filled with incriminating subpoenaed documents, but no punishment of the perpetrators on the books. The only pertinent question here is why hasn’t it happened?
Reading this now, I wonder why the approval was so broad ["management of the manifestations of psychotic disorders"] to start with. It was twenty years ago, and I think that’s the way most people were thinking about the antipsychotics then, but even at that, it seems like a blanket approval and we know it’s exactly what Janssen had in mind. To quote sales rep Tone Jones from the Texas trial, "You can’t be a billion dollar drug in a 1% market." Schizophrenia is that 1% market, and Janssen had its sights set much higher. In a later communication, the FDA confirmed the breadth of the Approval, but cautioned about the elderly once again, and insisted that Janssen note that the studies were done in short-term Schizophrenia trials. Then, in 1999, the FDA reviewed what Janssen was actually doing and issued a fairly firm "halt" order:
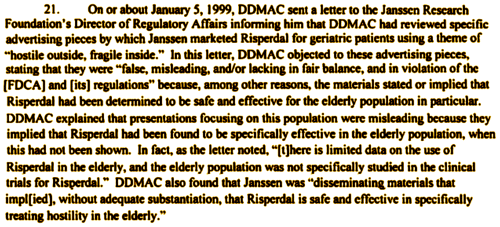
DDMAC = Division of Drug Marketing, Advertising and Communication [FDA]
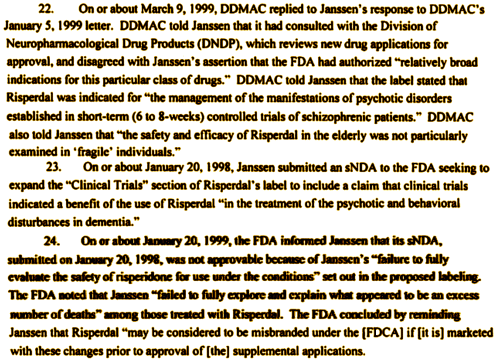
The back story here is that Janssen was cutting all kinds of deals to get Risperdal on the formularies and prescribed in beaucoups nursing homes. I’m going to leave you to look at that part yourself, but think Omnicare and think really big money. The FDA Approval probably was too broad in the first place. I think they were thinking about adults, where antipsychotics used in Schizophrenia have long been used for other conditions – like Acute Mania. But the FDA left a crack in the door for treating kids and the aged, and Janssen was racing through it by this time. When cautioned, Janssen pushed back, relying on the original wording, but they had also played it straight and applied for approval in the elderly. The FDA said "no," and brought up unexplained deaths:
So by 1999, the FDA had said "no" to dementia and had issued a threat. Selling Risderdal® as a treatment in dementia was not okay. Maybe there was a loophole to start with, but no longer. In fact, in March 2002, the FDA changed the approved indication to "treatment of Schizophrenia [see item 26 in the complaint].
What happened next is easily summarized [see pages 9 and 10 in the complaint]. In March 2003 the FDA requested a warning on the label about increased risk of stroke in the elderly, and added another in November, 2003 about Diabetes. In December, 2003 the FDA approved Risderdal® for short term treatment of Mania. Finally, in April 2005, the FDA required a Black Box Warning for all Atypical Antipsychotics, highlighting the risk of increased mortality in elderly patients with dementia-related psychosis. And the following month, they turned down yet another request for Risderdal®’s use in the elderly, this time in Alzheimer’s disease.
In summarizing, the FDA cut them some slack and didn’t complain about Janssen’s opportunizing on a loophole. What they don’t say is that Janssen was pushing Risderdal® for agitation, hostility, psychosis, etc, all the while getting it onto eldercare facilities’ formularies. In many places, Risderdal® was almost a routine drug in these institutions. Janssen was raking in the money from this enterprise throughout this whole story.
The behavior of Janssen was unaffected by these actions of the FDA. Their marketing Risderdal® for the elderly, if anything, escalated as the FDA continued to try to get it under control.
They recommended Risderdal® for non-psychotic behaviors for which it was never approved, and continued to push it for psychotic symptoms after the drug’s indications were refined to specifically discourage this use. And they knew what they were doing the whole time.
I apologize for the length of this post. I just couldn’t think of any way to shorten it. There are several points I’m trying to make. Janssen knew what it was doing. The FDA may have been slower than we would’ve have liked, but they were clear – and Janssen just kept on going. The dangers of Risderdal® to elderly patients became increasingly clear – and Janssen just kept on going. When things were coming to a head: the FDA was after their Eldercare plans; Allen Jones had filed his TMAP suit; the man in charge of Risderdal®, Alex Gorsky who headed the Risderdal® operation, had exited to Europe – and Janssen just kept on going. Nothing stopped them and this settlement won’t stop them if they’re in a similar situation in the future. The money is just too big. So I stand by "There’s just not enough honor in the marketplace to get the job done" and there never will be. And whatever we are doing now isn’t enough to get the job done either. The FDA and the DOJ gave it the old college try here.
-
First, in our negotiations with the pharmaceutical industry [and the KOLs], I’m closer to scorched earth than on any other issue I can think of. I’m not a scorched-earth kind of guy, but it’s clear that no matter what they say or even what they mean now, in the heat of battle when they can smell the profits, this story is typical of how they act – over and over – all of them. It’s not a question of trust. It’s evidence based!
-
Second. This story is missing teeth. Put the executives in jail [and the KOLs]. Take all the ill-gotten profit instead of a piece. Pull the drug’s patent protection. Do whatever it takes to stop the game playing with people’s health and lives. When we work with adolescents, we try to rehabilitate them, but at some point we throw in the towel, and it’s off to juvey. Our jails aren’t that overcrowded. There’s room for plenty of these guys…




What we are talking about is recidivism. Clinicians recognize that this trait is associated with sociopathy and narcissism (sense of entitlement). When the two co-occur, watch out! Juvey isn’t enough to hold ‘em…
It would take just a single individual’s criminal conviction (a CEO) to put a stop to most of this..
I would think someone would have started a Petition to the US Department of Justice demanding criminal prosecutions of individuals at re-offending companies. A draft of something like that could be written about as emotionally engaging as needed. One would think hundreds of thousands of people or more would sign..
“Johnson and Johnson’s Janssen Pharmaceuticals subsidiary from 1993 through the first three months of 2008: 1,207 children given Risperdal suffered serious problems, including 31 who died. Among the deaths was a 9-year-old with attention deficit problems who suffered a fatal stroke 12 days after starting therapy with Risperdal. At least 11 of the deaths were children whose treatment with Risperdal was unapproved by the F.D.A.” – 2008
http://www.nytimes.com/2008/11/19/health/policy/19fda.html
Everyone’s too afraid to take on the ‘Cartel’ though.
If the Department of Justice isn’t already prosecuting individuals, there’s probably a good reason. Why exactly though; I wonder where exactly one would even need to start?
I found this link of interest as it shows how the majority of drug ads are misleading or false.
http://pharmagossip.blogspot.com/2013/09/tv-drug-ads-mostly-false.html?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+blogspot%2FDlJuM+%28PharmaGossip%29
In my own experience I was shocked that over a decade ago when looking for a doctor I found doctors reciting verbatim the drug ads I had seen on television and in print. The results were doctors were more than willing to prescribe medications based on false information, information that would not be corrected by the drug companies if found to be false or misleading.
Doctors do not read the literature, but instead are influence by the soothing words of the sales reps who will tell them the warning is just for those few patients who will respond in a negative manner.
Pharma considers this sales and in any other industry it would be considered fraud. A few felony convictions would go a long way to at least slowing down this criminal activity. The time in Club Fed is not as important as with a felony you loose your legal ability to lead a large company, or hold any number of licenses. Only when people find themselves unemployable will there be any consideration to change behaviors.
Steve Lucas
Steve, You state: A few felony convictions would go a long way to at least slowing down this criminal activity. The time in Club Fed is not as important as with a felony you loose your legal ability to lead a large company, or hold any number of licenses. Only when people find themselves unemployable will there be any consideration to change behaviors.
I concur wholeheartedly . . . but I would add that now, since “corporations are people, my friend,” that the death penalty–the inability for a corporation OR ANY OF ITS SUBSIDIARIES to do business with the federal government (Medicare, Medicaid, VA)–would be deserved and easily hung on those corporations already operating under “corporate integrity agreements” that have allowed them (supposedly) one free pass. [BTW, some corporations are continuing to do business with two or three CIAs in place.]
I reference this post in my post for today. You can read it at the link below if you so desire.
http://eraycollins.postach.io/chump-change-update
Melody, good point. My post yesterday turned into a rant suggesting revocation of corporate charters. Seems a lot more sensible though when a company is operating with multiple corporate integrity agreements and still offending.
Mickey, I have a question regarding item 24 in the complaint — specifically, the “excess number of deaths” — were these deaths only in elderly patients, or were they from the initial trials? Regardless, are these numbers published anywhere? I once read the original Risperdal NDA and if I’m not mistaken it claimed there were NO deaths during the trials.
I would also like to know the answer to CODUFilm’s question about elderly deaths.