I really never took a business course or thought about marketing before I started writing this blog. I don’t even bargain with car salesmen. But I’ve learned a few things in the last couple of years. I didn’t understand indication creep until a couple of years ago. I was sitting in a too-hot courtroom trying not to nod off and the witness [Tone Jones] said "You can’t be a billion dollar drug in a 1% market" [the prevalence for Schizophrenia is 1%]. I came alert with an AHA! experience. That’s why once they get FDA Approval, they start going for other indications. Here’s how that indication race looked a few years ago with the Atypical Antipsychotics [the year Latuda® came on the scene]:
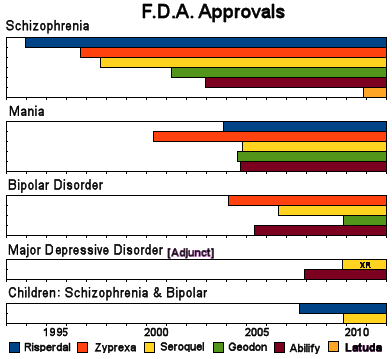
Without other diagnosis·specific indications, they can’t legally advertise. This is all pretty crazy, because nobody thinks these drugs are diagnosis·specific – at least nobody I know. But that’s the way the game has been played. Get the drug approved for a diagnosis, then spend the years of its patent life trying to get other indications for new ad launches. Towards the end of the patent, be sure to have a study or two in kids so you can get a pediatric extension. If it’s a blockbuster, try to get a long acting version approved [Paxil CR®, Seroquel R®] or maybe a purified isomer [Lexapro®, Focalin®] or even a metabolite [Pristiq®].
 But back to indication creep. According to the NIMH site, the prevalence of Bipolar Disorder is between two and three percent and the majority of episodes are depressive, so they add a market at least as big as the one they had. And we all know that Bipolar Disorder is way over·diagnosed. Let me say that one more time, Bipolar Disorder is way over·diagnosed. So their market grows even more because [did I mention] Bipolar Disorder is way over·diagnosed. And they covered their bases by the pair of studies – both monotherapy and adjunctive therapy, making it a clean sweep. But Johanna’s comment to the last post points out a more subtle marketing ploy that is probably even more lucrative:
But back to indication creep. According to the NIMH site, the prevalence of Bipolar Disorder is between two and three percent and the majority of episodes are depressive, so they add a market at least as big as the one they had. And we all know that Bipolar Disorder is way over·diagnosed. Let me say that one more time, Bipolar Disorder is way over·diagnosed. So their market grows even more because [did I mention] Bipolar Disorder is way over·diagnosed. And they covered their bases by the pair of studies – both monotherapy and adjunctive therapy, making it a clean sweep. But Johanna’s comment to the last post points out a more subtle marketing ploy that is probably even more lucrative:Funny you should mention the glossy-magazine-advertisement bit. I was just reading People Magazine yesterday, and came across a two-page spread advertising Latuda for bipolar depression (featuring a picture of a nice-looking 20-something woman with a vaguely hopeful expression on her face).
Worse yet, the “safety information” had been carefully worded to repeatedly refer to Latuda as an “antidepressant.” People will be offered this drug for no better reason than “my antidepressant doesn’t seem to be working” — and will have no earthly idea what they are fooling around with.

And depressed people began showing up in the clinic on Seroquel XR® being used as an antidepressant [with none of the criteria to meet these diagnoses except a depressed mood AKA unhappiness]. Now as Johanna discovered, that same strategy appears to be being picked up by Latuda®. The People Magazines in my doctor’s office are frayed and from an earlier historical epoch, so I hadn’t realized that Latuda® was being advertising there – but I’m not surprised. I don’t exactly know what to call this variant of indication creep – maybe grammatical sprawl.
The formal linking of these drugs to a DSM diagnosis based on clinical trials sounds good on paper, but in truth, it is not easy to defend – yet it’s the law of the land. But if only people actually meeting the DSM criteria were put on the drugs, we wouldn’t have anything like our current problem. I doubt that many people get a diagnosis of Schizophrenia made casually. But diagnostic precision goes way downhill after that. I mentioned that Bipolar Disorder is way over·diagnosed, but it doesn’t hold a candle to Major Depressive Disorder which is often diagnosed by a report that "I’ve been depressed" with little else. So any way that a pharmaceutical company can get the word depression into an ad is a major marketing success. I expect they were cheering at Sunovion when this months American Journal of Psychiatry came out.
Now comes the indication march proper [already in place well before the initial F.D.A. Approval]:
Sunovion® Latuda Phase III Clinical Trials NCT ID Title Recruit StartDate NCT00868699 6-week Study, Bipolar I Depression (Monotherapy) YES 04/2009 NCT00868959 24-week Extension Study, Bipolar I Depression YES 04/2009 NCT00868452 6-week Study, Bipolar I Depression (Add-on) YES 04/2009 NCT01284517 6-week Study, Bipolar I Depression YES 11/2010 NCT01358357 Bipolar Maintenance Adjunctive to Lithium or Divalproex YES 06/2011 NCT01421134 MDD With Mixed Features – Flexible Dose YES 09/2011 NCT01423253 MDD With Mixed Features – Extension Not Yet 09/2011 NCT01423240 MDD With Mixed Features Not Yet 10/2011
They’re following in the footsteps of some of the greats, well warned about the shoals of false advertising and other sleaze by the antics of their predecessors. Their problem – the generics are here. Even just plain Seroquel went off-patent this week – so, at $14+/pill for Latuda, it’s going to be an uphill climb. What they have going for them is that it seems to be weight neutral [so far]. What’s on the downside? It’s a decidedly weak sister [efficacy-wise]; it has an annoying EPS profile; and maybe, just maybe, people are finally tired of over-medicating and being over-medicated. But at Sunovion, hope springs eternal…
| Sunovion® Latuda Phase III Clinical Trials | ||
| NCT ID | Title | Status |
|
|
||
| NCT00868699 | 6-week Study, Bipolar I Depression (Monotherapy) | Done |
| NCT00868959 | 24-week Extension Study, Bipolar I Depression | Done |
| NCT00868452 | 6-week Study, Bipolar I Depression (Add-on) | Done |
| NCT01284517 | 6-week Study, Bipolar I Depression | Done |
| NCT01358357 | Bipolar Maintenance Adjunctive to Lithium or Divalproex | Recruiting |
| NCT01421134 | MDD With Mixed Features – Flexible Dose | Recruiting |
| NCT01423253 | MDD With Mixed Features – Extension | Done |
| NCT01423240 | MDD With Mixed Features | Withdrawn |
As you point out these are FDA rules regarding indications.There certainly is a marketing angle but on the other hand it may also reflect clinical reality. There are also potential advances at the level of a better overall medication with a better side effects profile. Anyone who has been around for decades has probably seen some of the last state hospital patients leave those facilities. It was not uncommon to see people with bipolar disorder stabilized on chlorpromazine monotherapy discharged and function well provided that they could take the medication. And yet for decades all of that prescribing was “off label”. I think it is preferable to have some evidence for prescribing for a specific indication even if it can be construed as indication creep.
The other issue is that in some cases it results in a major advance. The example I would give there is duloxetine and its various pain indications. In the chronic pain and addiction field it can be successfully used to treat neuropathic pain that was treated with opioids. It can also be used to treat back pain instead of chronic NSAID use (a much higher risk therapy). It has also been vetted by neutral party – The National Institute for Health and Care Excellence (NICE) in the UK where it is incorporated in their care recommendations for chronic neuropathic pain.
George,
I don’t disagree with your basic point. Both of your examples are situations where chronicity is part of the picture – and with chronic illness, we’re in the business of management. It is the skilled doctor who can optimally treat the symptoms of chronic illness without hurting people down the road. The other point from your example I’ve also seen – that Manic Depressive Illness is rarely limited to discrete episodes of Mania and Depression as we were once taught. In the spaces in between there is often difficult symptomatology to manage. And many patients use Alcohol or street drugs which isn’t a very good choice. In the case of Cymbalta®, or for that matter Neurontin®, patients say it helps. And neuropathic pain is particularly maddening, because it’s always there. So the issue is really the effects of long term use.
My complaint about Lurasidone® is that I don’t believe them. When I looked at it initially, the published article glowed, but when I read the FDA Medical Report, the warm glow evaporated. The AJP article made it sound like the Atypical Antipsychotic people had been looking for. No metabolic problems, EPS lite [at least lighter than the FGAs], efficacy lite but present. But that was short term and the FDA reviewer was pretty clear [with evidence] that it needed more thought. So I’m not willing to trust these articles.
Back to the point about diagnosis-specific indications. I don’t think there’s anything disease specific about using these medications in Bipolar Disorder. If you or I decide to use them, that’s our business, but I would want us to be scientifically informed about the long term consequences. I object, however, to saying that they are indicated in the “disease.”
And I am not sure I believe them when they promote duloxetine, even with its NICE backing. Funny how this drug came on to the scene as its fore fathers were going off patent. According to David Healy, duloxetine was a drug in search of an indication (initially developed to treat urinary incontinence). Gabapentin was the subject of massive over promotion as well. The studies of pain are as subject to the kind of biases who know so well. I can not provide you right now with the kind of documentation you might rightfully request, but I doubt we will be looking in back in 5 or 10 years and see this as some major medical advance.
This reminded me to approach all company sponsored research on expensive new drugs with a skeptical eye. I’d almost forgotten that Chicago’s despicable Dr. Reinstein once published research on Seroquel that actually claimed it could help patients lose weight.
http://articles.chicagotribune.com/2009-11-11/news/0911100746_1_antipsychotic-drug-psychotropic
That didn’t turn out so well for Chanile Hayes, a young Chicago woman he treated for a “nervous breakdown.” Seroquel caused her to literally double in size, and develop diabetes as well.
“She found it odd, she said, when Reinstein told her that taking Seroquel would help her lose weight. ‘I couldn’t understand why he wasn’t taking it because he was a plus-sized man himself,’ said Hayes, now 37. She is one of thousands of people nationwide suing AstraZeneca on allegations it concealed Seroquel’s links to weight gain and diabetes.”
Good point, Ms. Hayes. If 25% of Americans really are “living with mental illness,” why don’t we see more psychiatrists partaking of their own “safe, effective and well-tolerated” drug cocktails?
How effective is duloxetine? These polls of patients show:
– Cymbalta dead last among 85 treatments for fibromyalgia http://curetogether.com/blog/2011/08/10/patients-say-fibromyalgia-drugs-make-things-worse-rest-is-best/
– Cymbalta dead last among 35 treatments for neuropathy http://curetogether.com/blog/category/research-findings/
– Cymbalta (and Wellbutrin) very mediocre among 83 treatments for depression (Effexor and Paxil barely effective) http://curetogether.com/blog/category/research-findings/ — exercise, pets, art therapy, talk therapy rate much higher.
As for Latuda, doesn’t it seem odd that if it really causes less blood sugar disruption than other atypical antipsychotics, all Sunovian needs to do is trumpet this to take over the market? And yet not a peep about this huge advantage. Either they couldn’t find a KOL to front this claim or they’re not making it because even they have a sneaking suspicion it’s not true.
Instead, they’re doing mass-market advertising to capitalize on the belief of many people — fostered by 20 years of drug company propaganda and sloppy diagnosis — that if they have more than one emotion, they’re bipolar.
I also noticed the emphasis on the word “antidepressant” in Latuda TV ads. There must be some manipulation of the recording to make it stand out in the warnings fast-talk at the end.
Sorry, the complete links for
Cymbalta and neuropathy http://curetogether.com/blog/2011/08/16/neuropathy-study-results-800-people-rate-35-treatments/
Cymbalta and depression http://curetogether.com/blog/2011/05/03/23-surprisingly-effective-treatments-for-depression-one-year-later/
They are trumpeting the notion that it has less impact on metabolic parameters. I must get one phone a week from the local rep (no I do not talk to him). Has anyone see published results on the 24 week study? I would like to see the numbers for that. Aripiprazole is supposed to be “weight neutral” but I have some people who have gained considerable amounts of weight on it.
But Alto gets at where the “creep” occurs – it is in the flexibility of how we apply the label.
It seems before anyone with fibromyalgia is prescribed antidepressants, they should be screened for sleep breathing disorders.
http://www.practicalpainmanagement.com/pain/myofascial/fibromyalgia/sleep-apnea-patients-fibromyalgia-growing-concern
Unfortunately, my cynical brain doubts this will happen since psych meds are the answer to everything on this earth.
I’m sure there’s going to be a full-court press on doctors marketing-wise to get Sunovian into profit on Latuda. And you know what? It will work, because doctors still haven’t learned the lesson of the last 20 years of psychiatric drug oversell.
You would think, if the drug had merit on its own, aggressive marketing would not be necessary. Which is why I avoid taking any drug that’s been advertised on TV.
I hope this is not an ad hominem attack or proselytising, but maybe if we stopped referring to human suffering as “difficult symptomology” we might have more compassion and be less apt to drug people. Legal addiction to drugs that doctors prescribe is just as soul-killing as being addicted to street drugs. A prescription pad does not change the fact that psychiatric chemicals affect the body on the same way as drugs that Big Med does not approve of (or profit from.)