 We often use cracks as a metaphor: unwanted things [like weeds] shooting up through the cracks or things disappearing by falling through the cracks. The last post [kudus to AL·AK·AR·ID·IN·NC·OK·RI·UT·WI…], is an industry attempt to insure a crack in the new Sunshine Law – drug reps handing out reprints from peer reviewed journals to doctors without declaring industry’s part in the process. This is a huge crack that has allowed them to give out selected reprints [often from industry-sponsored clinical trials] as a way of advertising their wares in the guise of legitimate science. It has been a very effective marketing tool, and they’re afraid that making it public will make it less effective [let’s hope].
We often use cracks as a metaphor: unwanted things [like weeds] shooting up through the cracks or things disappearing by falling through the cracks. The last post [kudus to AL·AK·AR·ID·IN·NC·OK·RI·UT·WI…], is an industry attempt to insure a crack in the new Sunshine Law – drug reps handing out reprints from peer reviewed journals to doctors without declaring industry’s part in the process. This is a huge crack that has allowed them to give out selected reprints [often from industry-sponsored clinical trials] as a way of advertising their wares in the guise of legitimate science. It has been a very effective marketing tool, and they’re afraid that making it public will make it less effective [let’s hope].
 But cracks go both ways, letting things in and out. And one thing about things falling through the cracks, you don’t necessarily see it happen. You don’t know that something was there and then disappeared, it’s an empty space that doesn’t show. With snowfall on a deck, you can see the absences – the cracks. With pharmaceutical clinical trials, unless you know how to look, unpublished trials aren’t apparent.
But cracks go both ways, letting things in and out. And one thing about things falling through the cracks, you don’t necessarily see it happen. You don’t know that something was there and then disappeared, it’s an empty space that doesn’t show. With snowfall on a deck, you can see the absences – the cracks. With pharmaceutical clinical trials, unless you know how to look, unpublished trials aren’t apparent.
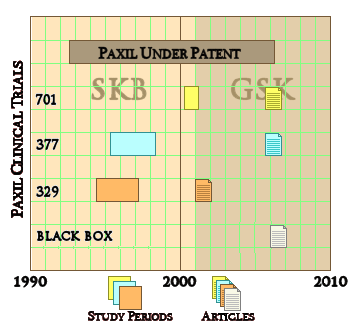 SmithKlineBeecham/GlaxoSmithKline gave us a classical example with the infamous Paxil® Study 329. They actually did three Clinical Trials [701, 377, 329] on Paxil® in adolesence, all completed before study 329 was published in 2001. That was the reprint handed out by the reps, promoting "off-label" use. The other two studies [701 & 377] were published after Paxil went off-patent 5 years later [as a CYA move]. Those studies were so negative that no amount of jury-rigging could save them. So, through the cracks they tumbled.
SmithKlineBeecham/GlaxoSmithKline gave us a classical example with the infamous Paxil® Study 329. They actually did three Clinical Trials [701, 377, 329] on Paxil® in adolesence, all completed before study 329 was published in 2001. That was the reprint handed out by the reps, promoting "off-label" use. The other two studies [701 & 377] were published after Paxil went off-patent 5 years later [as a CYA move]. Those studies were so negative that no amount of jury-rigging could save them. So, through the cracks they tumbled.Pharmafile04/03/14Half of all trials registered on a US clinical study database are not being published in public journals.This is according to a new report from Thomson Reuters Cortellis Clinical Trials, which found that of 600 studies chosen randomly from Clinicaltrials.gov – only 50% had their results published in a journal. ClinicalTrials.gov is the main registry and results database of publicly and privately supported clinical studies of human drug trials conducted around the world. The report called ‘Developments in Clinical Trials’, notes that lack or ambiguity in reporting clinical outcome achievement “could potentially be detrimental to clinical healthcare improvements”. It also found that some of the trials taking place today not only go unpublished, but also unregistered. “This might reduce a patient’s potential to find a suitable treatment, as well as lower the capability of the trial to include an appropriate and representative patient cohort,” the authors say.
This comes at a difficult time for the industry as in Europe new rules by both the European Medicines Agency and the European Parliament are set to be debated over the coming weeks. In fact on 3 April the Parliament is set to approve a new directive that will require anyone running a clinical trial to register it and publish a summary of results in a publicly accessible EU database. Currently these types of data are stored on a database that can only be accessed by the EMA. On top of this, full clinical study reports [CSRs] are also expected to be published following marketing authorisation of the product, or if the marketing authorisation is withdrawn.
This will be enforced by fines for any company or group that doesn’t adhere to the new transparency rules. But the industry has been fighting hard against this, arguing that it can self-regulate when it comes to releasing data. The UK pharma lobby group the ABPI said recently that new analysis – which it commissioned – found that more than three-quarters [76%] of all industry-sponsored clinical trials for new medicines recently approved by the Agency EMA had ‘some results disclosed’ within a year of completion or of regulatory approval. It also found that rates of disclosure have ‘continued to rise’ and almost nine out of 10 [89%] of these trials had disclosed results by 31 January 2013. But these data have been criticised by the UK transparency campaign group AllTrials, which says that independent analysis shows the rates of disclosure remain low.
The AllTrials initiative has also maintains that only half of all research studies are published, with those that have positive results appearing twice as frequently as those with negative results. These latest data from Thomson Reuters Cortellis will only serve to corroborate the stance taken by AllTrials, and fuel greater discussion over this divisive issue ahead of the European debate on the topic next month.hat tip to pharmagossip…
 To my way of thinking, cracks in regulations have been of major import to the whole history of deceptive marketing practices of the pharmaceutical industry. David Healy’s under-read Pharmageddon catalogs the story of how regulations that were designed to be reform measures have actually been turned into marketing tools by finding the cracks and exploiting them. And who would’ve thought that a company would go to all the trouble and great expense to just put it in a file drawer and let it collect dust. The FDA regulations have a crack big enough to drive a truck through. To prove efficacy and get approved, you have to have two well-conducted positive studies – a generous concession to industry. But what happened was that if the drug had any effect at all, no matter how weak, it could be turned into approval by doing a bevy of clinical trials and submitting two positive ones and not publishing [or mentioning] all of the failures. It looks like Roche was able to exploit that crack with the blockbuster Tamiflu® to the tune of billions.
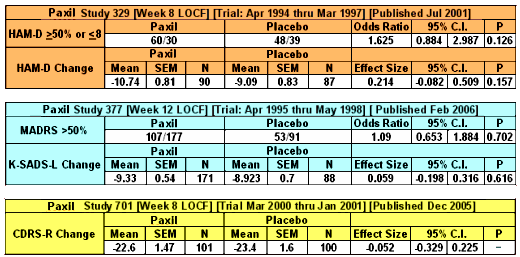
To my way of thinking, cracks in regulations have been of major import to the whole history of deceptive marketing practices of the pharmaceutical industry. David Healy’s under-read Pharmageddon catalogs the story of how regulations that were designed to be reform measures have actually been turned into marketing tools by finding the cracks and exploiting them. And who would’ve thought that a company would go to all the trouble and great expense to just put it in a file drawer and let it collect dust. The FDA regulations have a crack big enough to drive a truck through. To prove efficacy and get approved, you have to have two well-conducted positive studies – a generous concession to industry. But what happened was that if the drug had any effect at all, no matter how weak, it could be turned into approval by doing a bevy of clinical trials and submitting two positive ones and not publishing [or mentioning] all of the failures. It looks like Roche was able to exploit that crack with the blockbuster Tamiflu® to the tune of billions. In the case of Paxil® in adolescent depression, besides the not-publishing crack, they found some other cracks by messing with the protocol and omitting some essential analyses – turning Paxil® Study 329 into something they thought they could get away with calling a positive outing. It wasn’t really close. Here are the endpoints of the Primary Efficacy Variables in all three studies:
Are these practices of slipping in and out of the cracks just something they’ve happened onto? I don’t think so. For example, in kudus to AL·AK·AR·ID·IN·NC·OK·RI·UT·WI…, it looks to me like they’re trying to actively maintain a crack in the system of the new Sunshine Act. And from where I sit, it looks to me like industry is doing everything in its power to put cracks in the inevitable Data Transparency in the future – cracks that can be exploited at some later date. For example, AbbVie’s lawyer Neal Parker was dancing as fast as he could to put some cracks in the EMA’s resolve to release trial data [a deal-breaker?…]. Industry and their organizations fought Data Transparency tooth and toenail, and when it became inevitable, they have continued to try to leave openings. The current compromise they’re offering is to allow access to Clinical Trial data via "independent" boards deciding about access based on the merit of the request.
I’m suspicious. Where’s the crack? or the potential crack? While their argument that they don’t want competitors seeing their stuff makes some sense, I don’t trust them and am suspicious of their "independent" boards. The best predictor of future behavior is past behavior, and their past behavior is pretty rotten. I got myself in trouble questioning J&J’s turning that function over to Yale. While I’m sorry I offended Dr. Krumholtz [a placemarker…, reassure us…, a patch of blue…], I still think this is no time to let down any guards. I don’t think all the cracks that Pharma has turned to their advantage are simply good fortune, I think they’ve created opportunities for marketing tricks even as reforms have been being put in place. And I’m still suspicious of what these "independent" boards will become somewhere down the road.
This makes me think of kudzu. Taking advantage of any crack until it covers everything in a thick impenetrable blanket.
Steve Lucas
remember, if it’s “SO GOOD” and he has to come OUT to sell it, there must be a crack.
GOOD work will sell itself. that’s the point of a good medical science experiment. hell i bet it’s why a bunch of specialists in a lab run by SUITs are frustrated with what they’re being FORCED to do.
Publisher’s Note
75 Years…Another Milestone [Free Access]
John S. Shelton, PhD
This year, The Journal of Clinical Psychiatry celebrates its 75th anniversary. Here, our Publisher, John S. Shelton, PhD, reflects on how much JCP has grown over the last 75 years to become what it is today—a top-ten psychiatric resource and educational provider of CME activities. He thanks you, our readers, for being the real heroes in our success story.
Read more at http://www.psychiatrist.com/private/article_wrapper.asp?art=2014/v75n02/v75n0205/v75n0205.htm
the little notice aspect of “healthcare reform” is that the status quo has been loosened