With all of this talk of Data Transparency in Clinical Trials, people in-the-know tend to talk in shorthand jargon, and it’s easy to get lost. And since what they’re talking about actually matters, this is a mini tutorial on what the acronyms mean:
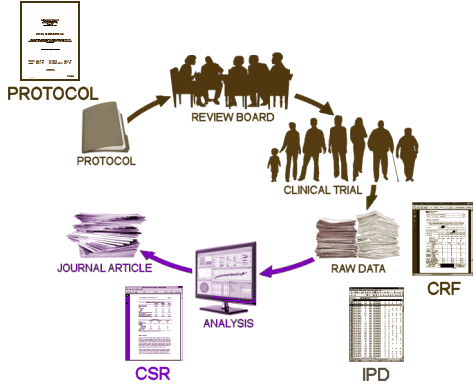
The Protocol is the nuclear document in a Clinical Trial, an a priori description that lays out everything about how the study is to be conducted and evaluated in detail. One part of those specs is to define the primary and secondary outcome variables, the methods used to analyze them, and the criteria for reaching conclusions. The Institutional Review Board is tasked with insuring that the study justifies doing a trial in humans, meets all the standards for human research, and is designed with sufficient power to answer the research question – pro or con.
 These days, the Clinical Trial itself is often spread among multiple sites, coordinated by a contracted CRO [Clinical Research Organization]. The subjects are randomized and double blinded so neither the study staff nor the subjects know what medication any subject is taking. Each of the tests and observations throughout the duration of the trial are recorded on a variety of individual Case Report Forms [CRFs] – the true Raw Data from a Clinical Trial. When the last subject enrolled completes the study, the stacks of CRFs are abstracted – turned into analysis ready tables called the Individual Participant Data [IPD], usually in an electronic format. This whole process takes place while the study is still fully blinded. The IPDs that tabulate the numeric or categorical scores from tests likely mirror the CRF data [but those cataloging Adverse Effects and other notations can lose something in translation].
These days, the Clinical Trial itself is often spread among multiple sites, coordinated by a contracted CRO [Clinical Research Organization]. The subjects are randomized and double blinded so neither the study staff nor the subjects know what medication any subject is taking. Each of the tests and observations throughout the duration of the trial are recorded on a variety of individual Case Report Forms [CRFs] – the true Raw Data from a Clinical Trial. When the last subject enrolled completes the study, the stacks of CRFs are abstracted – turned into analysis ready tables called the Individual Participant Data [IPD], usually in an electronic format. This whole process takes place while the study is still fully blinded. The IPDs that tabulate the numeric or categorical scores from tests likely mirror the CRF data [but those cataloging Adverse Effects and other notations can lose something in translation].
 When the blind is broken, the IPD datasets are ready for Analysis. That’s when the investigators, sponsors, and statisticians get their first look at the data sorted by treatment, and can apply all the tricks of their trade that tell us all if the study drug separates from placebo, the magnitude of the effect, and whether there are significant adverse effects from use. At this point, the whole process is turned into something called a Clinical Study Report [CSR], a long narrative document that tells the story from start to finish including the results. One thing to note, usually the relevant IPD tables are appended to the CSR, but that’s not always true. So it’s important when CSRs are being offered to find out if they have the unanalyzed data attached or not.
When the blind is broken, the IPD datasets are ready for Analysis. That’s when the investigators, sponsors, and statisticians get their first look at the data sorted by treatment, and can apply all the tricks of their trade that tell us all if the study drug separates from placebo, the magnitude of the effect, and whether there are significant adverse effects from use. At this point, the whole process is turned into something called a Clinical Study Report [CSR], a long narrative document that tells the story from start to finish including the results. One thing to note, usually the relevant IPD tables are appended to the CSR, but that’s not always true. So it’s important when CSRs are being offered to find out if they have the unanalyzed data attached or not.
One might think with a set of such rigidly structured procedures like this, everything ought to come off like clockwork – and yet we all know that it hasn’t. In psychiatry in particular, but to a lesser extent in the rest of medicine, Clinical Trials have become a major vehicle for misinformation, published in our top peer reviewed journals. Subtle manipulations in data analysis, presentation, and interpretation appear regularly in journal articles; many negative studies go unpublished, sins of omission and commission abound. So there has been a mounting consensus that making the raw data from Clinical Trials routinely available instead of the [hidden] property of the sponsors of the studies will allow independent vetting of the results. I’d like to take a look at these various proposals for Data Transparency, but it’s hard to figure exactly what’s being suggested. It gets lost in the acronyms. Thus my mini-tutorial to at least get the terminology straight.
Very good Mickey, very clear and useful. Tom.
excited bout the brain initiative or wat dr mickey & dr carroll?