Ever wonder how we knew if a treatment helped people or not before Randomized Clinical Trials? Seems almost imponderable to think about, but it was pretty easy. We just asked them. In this meta-analysis of the antidepressant trials of SSRIs in children and adolescents, Spielmans and Gerwig compiled the subjects own ratings of how they did, comparing the drug and placebo responses:
by Glen I. Spielmans and Katherine GerwigPsychotherapy and Psychosomatics. 2014 83:158–164.
Background: Recent meta-analyses of the efficacy of second- generation antidepressants for youth have concluded that such drugs possess a statistically significant advantage over placebo in terms of clinician-rated depressive symptoms. However, no meta-analysis has included measures of quality of life, global mental health, self-esteem, or autonomy. Further, prior meta-analyses have not included self-reports of depressive symptoms.Methods: Studies were selected through searching Medline, PsycINFO, and the Cochrane Central Register for Controlled Trials databases as well as GlaxoSmithKline’s online trial registry. We included self-reports of depressive symptoms and pooled measures of quality of life, global mental health, self-esteem, and autonomous functioning as a proxy for overall well-being.Results: We found a nonsignificant difference between second- generation antidepressants and placebo in terms of self-reported depressive symptoms [k = 6 trials, g = 0.06, p = 0.36]. Further, pooled across measures of quality of life, global mental health, self-esteem, and autonomy, antidepressants yielded no significant advantage over placebo [k = 3 trials, g = 0.11, p = 0.13].Discussion: Though limited by a small number of trials, our analyses suggest that antidepressants offer little to no benefit in improving overall well-being among depressed children and adolescents.
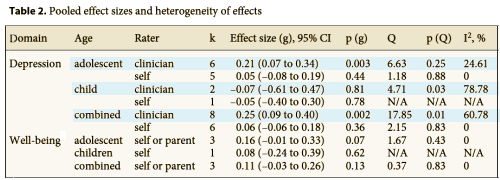
The table summarizes placebo versus treatment responses. Notice that none of subjects self rating showed a significant difference between SSRI and Placebo groups. Note also that in the two instances where the clinician ratings were significant, the Effect Sizes were only 0.25 and 0.21 which are in the small range. The results couldn’t be clearer. From the point of view of the subjects themselves, there was no perceived effect from the medication [which is, after all, the point of taking it].
Thank you for bringing this issue to the front burner, Dr. Mickey. The KOL corps have colluded in this gaming of the system for years. The regulators also share in responsibility for the de-emphasis of self-reports in clinical trials. Something has changed in our conceptual paradigm when a drug can be described as efficacious but the patients do not confirm that their lives are any better in respect of symptom burden or quality of life.
Leaving the patients to take things into their own hands. There is nothing scientific about the ratings at Ask a Patient and you read it at your own risk. However, I have found it presents a fairly balanced view of the effects of some of the medications I have been prescribed.
This site is what happens when you can’t trust or don’t receive information from those who business it is to have it. Not all that different than using the grapevine at work; sometimes you get burned with misinformation that is only gossip. However, if you never tap into the grapevine, you will ALWAYS get blindsided.
It would make sense to define Level 1 and Level 2 evidence of efficacy in antidepressant trials. Level 1 would be statistical evidence of efficacy by both observer ratings and by self-ratings. Level 2 evidence would be evidence from only one of these sources. The same principle could be carried over to trials in anxiety, obsessive-compulsive disorder, and any other disorder in which insight is not challenged by psychosis or delirium. The paternalistic stance of accepting only observer ratings for antidepressant efficacy should have been discarded long ago.
I’d be more interested in seeing a similar study of adult patients. Children and adolescents are notoriously bad assessors of their own well-being.