I thought we might be getting beyond these industry funded Clinical Trials of psychiatric medications being published in our peer reviewed journals, but they’re still there [though much less frequently]. This one is in the AJP published online last week ahead of print. It tests the effects of Seroquel XR® on patients with Borderline Personality Disorder using as the primary outcome measure the Zanarini scale [funded by AstraZeneca of course]. AstraZeneca pulled off some sleight of hand getting Seroquel XR® approved as Seroquel’s patent was running out. There’s nothing I know of that would suggest that Seroquel XR® would be any different from regular Seroquel®, but AstraZeneca continues with the Clinical Trials as if Seroquel XR® is something special:
by Donald W. Black, M.D. Mary C. Zanarini, Ed.D. Ann Romine, R.N. Martha Shaw, B.A. Jeff Allen, Ph.D. S. and Charles Schulz, M.D.American Journal of Psychiatry. 2014 Jun 27. [Epub ahead of print]
Objective: The authors compared the efficacy and tolerability of low and moderate dosages of extended-release quetiapine in adults with borderline personality disorder.Method: Ninety-five participants with DSM-IV borderline personality disorder were randomly assigned to receive 150 mg/day of quetiapine [the low-dosage group; N=33], 300mg/day of quetiapine [the moderate-dosage group; N=33], or placebo [N=29]. Total score over time on the clinician-rated Zanarini Rating Scale for Borderline Personality Disorder [“Zanarini scale”] was analyzed in a mixed-effects model accounting for informative dropout.Results: Participants in the low-dosage quetiapine group had significant improvement on the Zanarini scale compared with those in the placebo group. Time to response [defined as a reduction of 50% or more on the Zanarini scale total score] was significantly shorter for both the low-dosage quetiapine group [hazard ratio=2.54, p=0.007] and the moderate-dosage quetiapine group [hazard ratio=2.37, p=0.011] than for the placebo group. Among participants who completed the study, 82% in the low- dosage quetiapine group were rated as “responders,” compared with 74% in the moderate-dosage group and 48% in the placebo group. Treatment-emergent ad-verse events included sedation, change in appetite, and dry mouth. The overall completion rate for the 8-week double-blind treatment phase was 67% [67% for the low-dosage quetiapine group, 58% for the moderate-dosage quetiapine group, and 79% for the placebo group]. Participants who experienced sedation were more likely to drop out.Conclusions: Participants treated with 150 mg/day of quetiapine had a significant reduction in the severity of borderline personality disorder symptoms compared with those who received placebo. Adverse events were more likely in participants taking 300 mg/day of quetiapine.
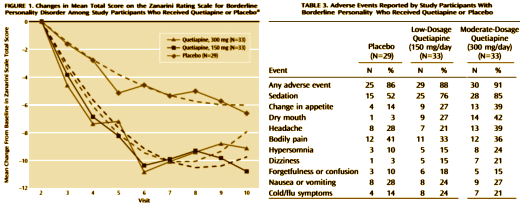
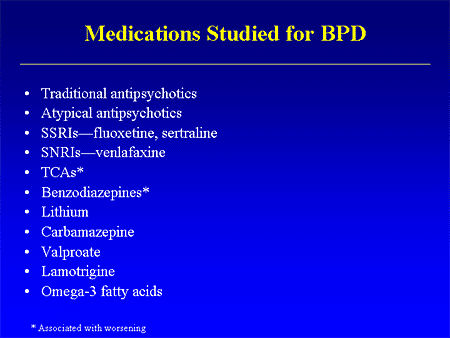
In a number of ways, this Clinical Trial embodies the story of the last thirty years. If there’s any solid evidence that there’s a biological antecedent to this disorder, I don’t know about it. Certainly nothing that would suggest that an atypical antipsychotic would be treatment other than as a "calmer downer" in an agitated crisis state. But Dr. Schulz has been trying various medications in this condition for a very long time. In an industry funded Medscape CME, he shows a slide of the drugs that have been tried:
The most recent Cochrane review [2010] says:
by Stoffers J, Völlm BA, Rücker G, Timmer A, Huband N, and Lieb K.Cochrane Database Systematic Review 2010 Jun 16;[6]:CD005653.…AUTHORS’ CONCLUSIONS: The available evidence indicates some beneficial effects with second-generation antipsychotics, mood stabilisers, and dietary supplementation by omega-3 fatty acids. However, these are mostly based on single study effect estimates. Antidepressants are not widely supported for BPD treatment, but may be helpful in the presence of comorbid conditions. Total BPD severity was not significantly influenced by any drug. No promising results are available for the core BPD symptoms of chronic feelings of emptiness, identity disturbance and abandonment. Conclusions have to be drawn carefully in the light of several limitations of the RCT evidence that constrain applicability to everyday clinical settings [among others, patients’ characteristics and duration of interventions and observation periods].
 And the Seroquel XR® study is no different, having no significant effect on core symptoms [speaking of significance, the higher dose Seroquel XR® was not statistically significant]. This study is a classic "experimercial" aiming towards fostering the off-label use of Seroquel XR® in this condition. This whole enterprise of doing Clinical Trials in Borderline Personality Disorder is not science based, more a shotgun approach to see what sticks. Here’s another one from four years ago, funded by Eli Lilly aiming to study Zyprexa in this group of patients, also by Dr. Schulz. Similar results:
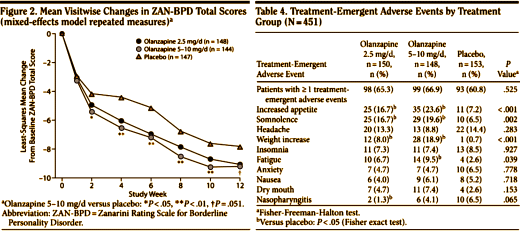
And the Seroquel XR® study is no different, having no significant effect on core symptoms [speaking of significance, the higher dose Seroquel XR® was not statistically significant]. This study is a classic "experimercial" aiming towards fostering the off-label use of Seroquel XR® in this condition. This whole enterprise of doing Clinical Trials in Borderline Personality Disorder is not science based, more a shotgun approach to see what sticks. Here’s another one from four years ago, funded by Eli Lilly aiming to study Zyprexa in this group of patients, also by Dr. Schulz. Similar results: by Mary C. Zanarini, EdD; S. Charles Schulz, MD; Holland C. Detke, PhD; Yoko Tanaka, PhD; Fangyi Zhao, PhD; Daniel Lin, PhD; Walter Deberdt, MD; Ludmila Kryzhanovskaya, MD; and Sara Corya, MDJournal of Clinical Psychiatry. 2011 72[10]:1353–1362.
Objective: To examine the efficacy and safety of olanzapine at low and moderate doses for the treatment of borderline personality disorder.Method: In this 12-week randomized double-blind placebo-controlled trial, 451 outpatients aged 18–65 years with DSM-IV borderline personality disorder received olanzapine 2.5 mg/d [n = 150], olanzapine 5–10 mg/d [n = 148], or placebo [n = 153]. The trial was conducted from February 2004 through January 2006 at 59 community-based and academic study centers in 9 countries [United States, Italy, Poland, Romania, Turkey, Chile, Peru, Argentina, and Venezuela]. The primary efficacy measure was mean change from baseline to last-observation-carried-forward endpoint on the Zanarini Rating Scale for Borderline Personality Disorder [ZAN-BPD] total score. Secondary measures included the Montgomery-Asberg Depression Rating Scale, the Modified Overt Aggression Scale, the Global Assessment of Functioning, the Symptom Checklist-90-Revised, and the Sheehan Disability Scale.Results: An overall mean baseline ZAN-BPD total score of 17.2 [SD = 4.9] indicated moderate symptom severity. Only treatment with olanzapine 5–10 mg/d was associated with significantly greater mean change from baseline to endpoint in ZAN-BPD total score relative to placebo [−8.5 vs −6.8, respectively; P = .010; effect size = 0.29; 95% CI, 0.06–0.52]. Response rates [response indicated by ≥ 50% decrease from baseline in ZAN-BPD total score] were significantly higher for olanzapine 5–10 mg/d [73.6%] versus olanzapine 2.5 mg/d [60.1%; P = .018] and versus placebo [57.8%; P = .006]. Time to response was also significantly shorter for patients taking olanzapine 5–10 mg/d than for placebo-treated patients [P = .028]. Treatment-emergent adverse events reported significantly more frequently among olanzapine-treated patients included somnolence, fatigue, increased appetite, and weight increase [all P values < .05]. Mean weight change from baseline to endpoint was significantly greater for olanzapine-treated than for placebo-treated patients [olanzapine 2.5 mg/d: 2.09 kg; olanzapine 5–10 mg/d: 3.17 kg; placebo: 0.02 kg; P < .001]. The overall completion rate for the 12-week double-blind treatment period was 65.2% [ie, 64.7% for olanzapine 2.5 mg/d, 69.6% for olanzapine 5–10 mg/d, and 61.4% for placebo].Conclusions: Olanzapine 5–10 mg/d showed a clinically modest advantage over placebo in the treatment of overall borderline psychopathology. This advantage in effectiveness should be weighed against the risk of adverse events [particularly weight gain], which were consistent with the known safety profile of olanzapine.
a·nach·ro·nism [ -n
-n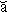 k
k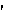 r
r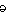 -n
-n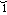 z
z
 m]
m]
 -n
-n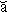 k
k r
r -n
-n z
z
 m]
m] n.
a thing or person that belongs to another, especially an earlier, time.
 It’s time for our journals to realize that they’re not roadside billboards and put an end to publishing articles whose primary purpose is advertising expensive drugs, off-label, particularly the major journals like the American Journal of Psychiatry. That’s just not what they’re for. That’s never been what they are for…
It’s time for our journals to realize that they’re not roadside billboards and put an end to publishing articles whose primary purpose is advertising expensive drugs, off-label, particularly the major journals like the American Journal of Psychiatry. That’s just not what they’re for. That’s never been what they are for…
Yawn… another experimercial. Notice the branding language even in the title: ‘low and moderate doses’… Sounds just like those direct-to-consumer advertisements that feature ‘moderate to severe psoriasis.’ And The American Journal of Psychiatry is suckered in. Memo to APA president Summergrad: fire everybody involved in the review of this crass article. Did it illuminate anything about the disorder? Silly me for asking.
I’d tread carefully on what is mentioned together; next thing you know, there will be a study of Seroquel XR® for psoriasis.
Seriously though, I don’t think I’ve seen a class of drug more in search of a disease to treat.
This link may be of interest:
http://davidhealy.org/we-are-the-ninety-nine-percent/?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%3A+DrDavidHealy+%28Dr.+David+Healy%29
Steve Lucas
I vomited all over my desk when I saw this in my copy of the AJP.
Com’n now, the University of Minnesota’s department of psychiatry is world renown for it’s clinical research. Just ask them. Why, they were the only site funded by AstraZeneca to conduct the cutting edge trial :The Use of Quetiapine (Seroquel) in the Treatment of Social Phobia: Public Speaking Environment. I probably should have popped a bad-boy Seroquel before even sitting down and typing this comment.
But you don’t get paid to treat Axis 2 disorders, so what’s the point of this whole exercise in the end? Besides, I have seen more complications in using psychotropics for primary Axis 2 disorders than any real benefits, so are outcomes really noting improvements in interpersonal functions, or, just measuring sedation, distraction, and avoiding as much weight gain and hyperglycemia as possible?
It is about getting it right, and how many patients with Axis 2 disorders are really ready to face the truth? Oh yeah, another “benefit” to Seroquel, distorting those truths!!!
Arby, that wouldn’t be anything new:
http://www.bonkersinstitute.org/medshow/thorazpsor.html
Gad Mayer,
Well, that’ll teach me. There truly is nothing new under the sun.
The website that hosts that image is pretty funny and fascinating in a creepy sort of way.
DTC advertising is selling disease, not the cure. This is why so many DTC treatments are for conditions that cant be tested for. “My Doctor diagnosed me with fibromyalgia” means “My Doctor doesn’t know what wrong with me either, including nothing”. These are narratives, which are memes.