I want to say some things about this article later…
by Mark KesselNature Biotechnology 2014 32[10]:983–990.
… but first, here’s a graphic and table to ponder:
| Date | Pharma | Fine [$M] | Infringement |
|
|
|||
| 02/2014 | Endo |
193 | criminal and civil liabilities arising from Endo’s marketing of the prescription drug Lidoderm [lido-caine]. As part of the agreement, Endo admitted that it intended that Lidoderm be used for unapproved indications and that it promoted Lidoderm to healthcare providers this way. |
| 11/2013 | J&J | 2,200 | criminal and civil allegations relating to illegal promotion of the prescription drugs Risperdal [risperidone], invega [paliperidone] and Natrecor [Nesiritide] for uses not approved as safe and effective by the FDA, the targeting of elderly dementia patients in nursing homes, and the payout of kickbacks to physicians and to the nation’s largest long-term care pharmacy provider, Omnicare. |
| 12/2012 | Amgen | 762 | criminal and civil charges that the company illegally introduced and promoted several drugs, including Aranesp [darbepoetin alfa], a drug to treat anemia. Amgen pleaded guilty to illegally selling Aranesp to be used at doses that the FDA had explicitly rejected, and for an off-label treatment that FDA had never approved. |
| Sanofi | 109 | Allegations that company gave doctors free units of Hyalgan [hyaluronate injection to relieve knee pain] to encourage sales, lowered the effective price by promising doctors free samples, while at the same time obtaining inflated prices for the drug from government programs by submitting false price reports. | |
| 10/2012 | Boehringer Ingelheim | 95 | Allegations that company promoted several drugs including Aggrenox [aspirin/dipyridamole], Atrovent [ipratropium], Combivent [Ipratropium/albuterol] and Micardis [telmisartan] for nonmedical ly accepted uses. |
| 07/2012 | GSK | 3,000 | Civil and criminal liabilities regarding misbranding of Paxil for treating depression in patients under 18, even though the drug had never been approved for that age group as well as failure to disclose safety information about Avandia to the FDA. |
| 05/2012 | Abbott | 1,500 | illegal promotion of Depakote [divalproex] in indications for which it had never been approved: schizophrenia and control of aggression and agitation in elderly dementia patients. |
| 11/2011 | Merck | 950 | illegal promotion of Vioxx as a treatment for rheumatoid arthritis before it had been approved for that use and misrepresentation of the drug’s heart safety to increase sales. |
| 04/2010 | Astra-Zeneca |
520 | Allegations of illegal promotion of Seroquel [quetiapine] for a variety of unapproved uses, such as aggression, sleeplessness, anxiety and depression. The company paid the fine but denied the allegations. |
| 09/2009 | Pfizer | 2,300 | Misbranding Bextra with "the intent to defraud or mislead," promoting the drug to treat acute pain at dosages the FDA had previously deemed dangerously high. Bextra was pulled from the market in 2005 due to safety concerns. The government alleged that Pfizer also promoted three other drugs illegally: Geodon [ziprasidone], Zyvox [linezolid]and Lyrica [pregabalin]. |
| 01/2009 | Eli Lilly | 1,420 | Off-label promotion of Zyprexa [olanzapine] to elderly populations to treat dementia. The US government also alleged that Lilly targeted primary care physicians to promote Zyprexa for unapproved uses and "trained its sales force to disregard the law." |
As we say in church: If you want to see what a person believes in, look at their check book.
Steve Lucas
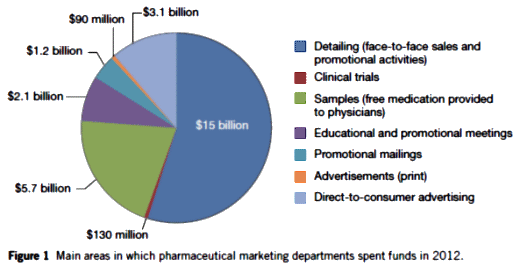
That’s a graph of how marketing departments spent money, not pharma spending in total. Why is marketing spending 130 m on clinical trials? That should all be subsumed under R and D. Obviously pharma spends more than that on clinical trails, it costs a billion and a half to get FDA approval for each drug. Which is too much no matter how you feel about any of the other issues we talk about here. Making them spend 3 billion and 30 years to approval isn’t going to make their lives or our lives any easier.
I would guess the clinical trials expense booked to Marketing represents faux-trial kickbacks to practitioners for ‘evaluating’ a drug, and maybe also some classic experimercials – where there is no serious scientific question.
The direct to consumer thing fascinates me. When you see those ads, you figure at first that there is no way they would be effective. Minutes of warnings and side effects over fields of daisies and butterflies and his/her bathtubs that somehow have working plumbing miles from civilization. But apparently they do work. Studies have shown that while the consumer is initially put off by the warnings, getting the bad news out there actually creates a sense of trust a few weeks later.
These numbers become extraordinary when you look at the DTC numbers vs. a 300M population.
Additionally we need to remember there are app. 624,000 physicians involved in patient care with only app. 209,000 out of that group involved in primary care.
We need to also remember that low income and charity clinics do not receive sales calls or free samples, thus increasing the pressure on those doctors who do see reps.
Being able to put pen to prescription pad is a very valuable commodity in the eyes of pharma.
Steve Lucas
Since when is the pharmaceutical industry concerned about its reputation?
The author of the article:
“Affiliations
Mark Kessel is chairman of the Foundation for Innovative New Diagnostics (FIND), counsel to Shearman and Sterling LLP and a founding partner of Symphony Capital LLP, New York, New York, USA.
Competing financial interests
The author declares no competing financial interests.”
• FIND
• Shearman and Sterling LLP
• Symphony Capital LLP
Steve,
Times are changing?
As Doctors Lose Clout, Drug Firms Redirect the Sales Call.
So now hospitals are going to dictate prescribing practices…its already happening I guess..
What is the function of a doctor in the future? Powerpoint sponge and chart monkey.
Anyone with a creative or critical thinking streak going into medicine is going to be gravely disappointed.
The formulary is the key to drug sales.
The question has been asked if medicine is being dumbed down by the use of computers. Check the box for conditions, get a diagnosis, and then go to the approved formulary for the prescription.
We already see pharma controlling the indicators and the approved standards of care. Their charge is not first do no harm, but sell.
As long as clinicians tolerate, if not abdicate, if not even greater replicate the profit agenda by all outside the providing of care realm, then nothing will be done to see a return to responsible and appropriate care outcomes.
It is so amusing, but moreso pathetic and annoying to listen to people tell me how Obamacare is going to do such wonderful things for healthcare, and then these same people complain about things like prescription authorizations or lack of provider access. Gee, the two issues of universal care and management of it are incongruent?
Nah, the ability to reason and problem solve seems to be incongruent these days. You want to profit, invest in oil, not blood!