by Delbert G. Robinson, Nina R. Schooler, Majnu John, Christoph U. Correll, Patricia Marcy, Jean Addington, Mary F. Brunette, Sue E. Estroff, Kim T. Mueser, David Penn, James Robinson, Robert A. Rosenheck, Joanne Severe,Amy Goldstein, Susan Azrin, Robert Heinssen, and John M. KaneAmerican Journal of Psychiatry. 2014; AiA:1–12; doi: 10.1176/appi.ajp.2014.13101355.
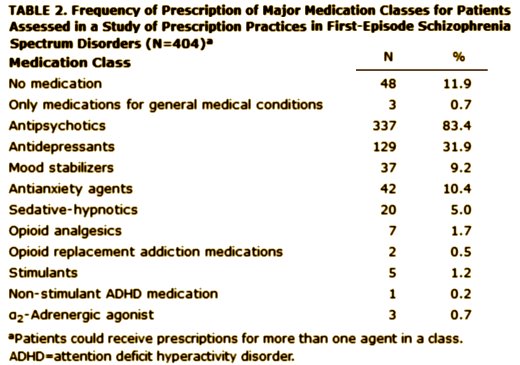
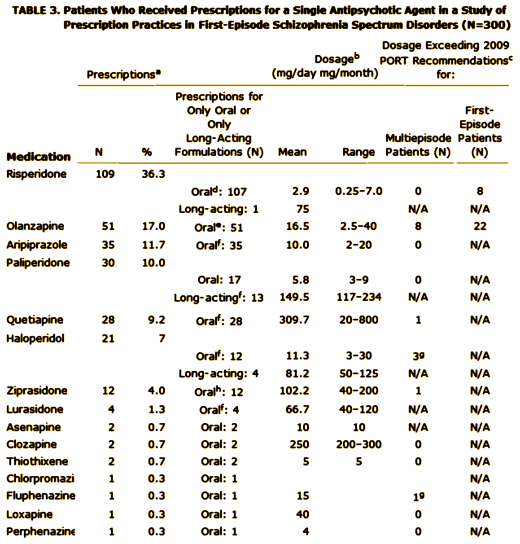
Objective: Treatment guidelines suggest distinctive medication strategies for first-episode and multiepisode patients with schizophrenia. To assess the extent to which community clinicians adjust their usual treatment regimens for first-episode patients, the authors examined prescription patterns and factors associated with prescription choice in a national cohort of early-phase patients.Method: Prescription data at study entry were obtained from 404 participants in the Recovery After an Initial Schizophrenia Episode Project’s Early Treatment Program [RAISE-ETP], a nationwide multisite effectiveness study for patients with first-episode schizophrenia spectrum disorders. Treatment with antipsychotics did not exceed 6 months at study entry.Results: The authors identified 159 patients [39.4% of the sample] who might benefit from changes in their psychotropic prescriptions. Of these, 8.8% received prescriptions for recommended antipsychotics at higher than recommended dosages; 32.1% received prescriptions for olanzapine [often at high dosages], 23.3% for more than one antipsychotic, 36.5% for an antipsychotic and also an antidepressant without a clear indication, 10.1% for psychotropic medications without an antipsychotic, and 1.2% for stimulants. Multivariate analysis showed evidence for sex, age, and insurance status effects on prescription practices. Racial and ethnic effects consistent with effects reported in previous studies of multiepisode patients were found in univariate analyses. Despite some regional variations in prescription practices, no region consistently had different practices from the others. Diagnosis had limited and inconsistent effects.Conclusions: Besides prescriber education, policy makers may need to consider not only patient factors but also service delivery factors in efforts to improve prescription practices for first-episode schizophrenia patients.
[reformatted to fit]
[truncated and reformatted to fit]
Harvard Mental Health LetterJUN 2010The PORT recommendations, issued in 1998 and first updated in 2003, were funded by the Agency for Healthcare Research and Quality and the National Institute of Mental Health. Researchers at the University of Maryland wrote the latest update after consulting with leading schizophrenia experts. In contrast to efforts like the American Psychiatric Association practice guidelines for schizophrenia and the Texas Medication Algorithm Project, which attempt to address the full range of situations clinicians encounter, the PORT review is more conservative in scope. The PORT authors limit their recommendations to those interventions that have been tested in randomized controlled trials…
Since the last PORT review, two large clinical trials have compared efficacy of first- and second-generation antipsychotics: the Clinical Antipsychotic Trials of Intervention Effectiveness [CATIE] and the Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study [CUtLASS]. Based on the findings of these studies, the PORT reviewers noted that in many cases, first- and second-generation antipsychotics are equally effective for treating schizophrenia.First-episode psychosis. The PORT review recommends using any antipsychotic except clozapine [Clozaril] and olanzapine [Zyprexa], because these drugs are most likely to cause significant weight gain and other metabolic side effects. Because patients experiencing psychosis for the first time are both more responsive to medications and more likely to have side effects, antipsychotics should be prescribed at doses that are lower — generally about half — compared with those recommended for patients with chronic schizophrenia.Relapse. Patients who initially responded to medication but suffer a relapse of symptoms have several options. The PORT team recommends any first- or second-generation antipsychotic other than clozapine, and stipulates that medication be prescribed at the lowest effective doses to reduce side effects. Choice of which antipsychotic to use depends on patient preference, past medication response, side effects, and medical history.Maintenance therapy. Studies that have followed patients with first-episode or chronic schizophrenia for one to two years have concluded that continuous maintenance antipsychotic treatment reduces risk of relapse. The PORT review recommends that intermittent maintenance therapy — a strategy of stopping antipsychotics until symptoms reappear or worsen — be reserved only for patients who refuse to continue taking an antipsychotic or for those who cannot tolerate the side effects. For patients with chronic schizophrenia, both first- and second-generation antipsychotics are equally effective at preventing relapse. During maintenance therapy, first-generation drugs may be used at lower doses than those required to treat the initial [acute] episode, while second-generation drugs can be prescribed at whatever dose was effective in the initial phase. Long-acting injectable antipsychotics provide another option in maintenance therapy, especially for patients who have trouble taking medication. The PORT review concluded that it is unclear whether injectable medications are any more effective than pills at preventing relapse, mainly because of a lack of randomized controlled studies….
"The guidelines linked on this page, excluding Major Depressive Disorder, are more than 5 years old and have not yet been updated to ensure that they reflect current knowledge and practice. In accordance with national standards, including those of the Agency for Healthcare Research and Quality’s National Guideline Clearinghouse, these guidelines can no longer be assumed to be current."
by Delbert G Robinson, Margaret G Woerner, Howard M Delman, and John M KaneSchizophrenia Bulletin. 2005 31[3]: 705-722.
Studies with first-episode populations offer the unique opportunity to examine the effectiveness and side effects of medications without the confounding effects of prior medication use. This review focuses upon studies of [1] treatment of the initial episode, [2] maintenance treatment issues, [3] recovery, and [4] side effects. Response rates for the initial episode are high with both conventional and new-generation antipsychotics. However, we lack data directly comparing the new-generation agents with one another for treatment of the initial episode, and data about options for patients with treatment resistance at illness onset are very limited. With the most commonly used pharmacological therapies, the course of early-phase schizophrenia is characterized by repeated relapses and a low rate of recovery. Medication treatment is also associated with a variety of side effects. Of particular concern for treatment of first-episode patients are the metabolic side effects with the new-generation antipsychotics because they occur rapidly, are very distressful to adolescents and young adults, and have long-term medical consequences. Available data support maintenance treatment to prevent relapse, but questions remain about the optimal duration of maintenance treatment, whether there are differences among the new-generation agents for maintenance treatment, and balancing the benefits of maintenance antipsychotics with their long-term side effects.
"The study found that about 40% of patients who’d been diagnosed with having psychotic experiences for the first time in their lives were being too heavily medicated right away."
First episode schizophrenia isn’t that hard to diagnose, so my assumption is that the patients enrolled in the RAISE study fit the bill for that diagnosis. Whether the medications they were on when they showed up are typical for the whole country or represent something else, it’s a bad showing. By my read, it’s worse than these authors say it is. 10% were on no psychoactive medication at all. The only reason I can think of that would explain that is that the clinicians knew they were going into the study and deferred treatment on that basis, but there’s nothing supporting that in the paper. Of the ones on antipsychotics, the big majority were on atypicals [SGAs] in spite of ample evidence that those drugs are no better than the older drugs [FGAs] and the side effects are really no less benign taken en masse. There’s no slightly reasonable explanation why so many were on Olanzapine, and in the higher dose range at that. It doesn’t take Guidelines to know that the weight gain/metabolic profile of that particular drug is a problem waiting to happen, particularly in first episode patients. You can see that in a waiting room. The author’s explanation for the too high dose being related to the treating chronic patients does make some sense relating to using Olanzapine and Haldol, the two drugs given in excess, but that doesn’t make it right. And a third of them taking antidepressants? That’s the kind of thing that happens when doctors equate the class nick-name "antidepressants" with what the drug actually does, and give them to anyone who says "I’m depressed." I know of no evidence that they help people with psychosis who say, "I’m depressed." That’s not who they’re for. We can do a hell of a lot better than what this study reveals. It’s tempting to see this result as a triumph of the "detail men" over science and good sense.
Gaebel et al. report results involving a subsample of first-episode patients participating in a 2-year randomized open treatment study comparing 3 medication strategies: continuous maintenance treatment, prodrome-based intervention, and crisis intervention. Prodrome-based intervention involved the reintroduction of antipsychotic medication as soon as prodromal symptoms [suspected predictors of impending relapse, e.g., restlessness, trouble sleeping, trouble concentrating, tension and nervousness, loss of interest or depression] occurred. Once restabilization was attained the antipsychotic drugs were again discontinued. Crisis intervention provided for the reintroduction of antipsychotic medication only in the case of a full relapse. Relapse rates during the 2-year period for first-episode subjects who completed the study were as follows: maintenance treatment, 38%; prodrome-based intervention, 42%; crisis intervention, 67%.
- a fabrication?…
- where’s the beef?…
- out of the loop…
- its effectiveness…
- another IRT prequel…
- on IRT, some comments…
- the manual…
The more meds, both number and quantity, is epidemic in all of medicine. I watch as friends go to the doctor for a simple condition and return with multiple medication and the result of that is predictable, drug interaction.
A friend went to France and three days into the trip had a heart attack and spent the next two weeks in the hospital. Returning home his cardiologist was pleased with his care and made no changes in medication.
Three months later his health is failing and the cardiologist is baffled until he asked what his GP was giving him. The GP had prescribed one medication at three times the recommended dose and added another drug that is never to be given with the first.
The cardiologist is now this person’s GP and is working minimizing medications and monitoring changes in his health. Much like the story here, less is more.
Today doctors of all types claim the rush of time only allows them to read a pharma prepared abstract or talk to a drug rep. This does not serve their patients or themselves well as prescribing issues combined with cost result in less than optimal results.
Or maybe that is the desired result as doctors tie patients to their practice as a functioning patient does not generate revenue.
Steve Lucas
All too common, Steve, which is why I think pharmacologists should have a role in prescribing and prevent a lot of dangerous interaction, overdoses, and otherwise contraindicated prescribing. It’s understandable that most MDs do not have the time, and even enough money, to keep up with even just all the medications and other interventions that are routinely used in their specialties.
I wound up on your site searching for the basis of the PORT recommendation that olanzapine not be used in first episode psychosis. This recommendation was new to me even though I should have seen it sooner. Nonetheless, I’m still trying to find some evidence to support the recommendation aside from opinions about the morbidity from weight gain – like time to hospitalization, mortality, etc. Whether appropriate or not, I am slightly biased towards olanzapine in some patients. Like you, I don’t like all of the drug company influence in the scientific literature as it muddies the waters. While reading the actual PORT recommendations, I looked at the disclosures:
R.W.B.: Data and Safety Monitoring Board member: Cephalon and Pfizer; Consultant: Abbott, Glaxo-Smith-Kline, Natixis Bleichroeder, Sanofi-Aventis, Schering-Plough; Advisory Board: Astra-Zeneca, Merck, Pfizer, Roche, Solvay Pharmaceuticals, Inc., Wyeth, Grant Support: Janssen Pharmaceutica; D.L.K.: Advisory Board: Bristol Myers Squibb, Janssen Pharmaceutica, and Solvay. Julie Kreyenbuhl, Jason M. Noel, Douglas L. Boggs, Bernard A. Fischer, Seth Himelhoch, Beverly Fang, Eunice Peterson, Patrick R. Aquino, and William Keller have no competing interests or financial support to disclose.
Maybe I’m missing it, but I didn’t find Lilly mentioned in there. While olanzapine is off patent now, the recommendation was made in 2009 and it is rare where I see a recommendation / guideline specifically discourage use of a particular drug unless there is good evidence to support the recommendation. At that time, it seemed to me that most efficacy studies seemed to slightly favor olanzapine. I couldn’t find anything explaining the reasoning in the PORT recommendation other than reference to a 2006 study by Robinson and that article concludes:
“A crucial unanswered question deserving investigation is whether
newer second-generation antipsychotics with lower propensity
to cause metabolic side effects might be the most
appropriate first treatments.”
Somehow that led to a recommendation in 2009, and now a study pointing out many psychiatrists are not following that recommendation.
On the issue of 10% un-medicated – is it perhaps the patient was not wanting to take any medication? I frequently encounter patients with psychosis who do not want to take medications.