New York TimesBy Richard A. Friedman, M.D.January 8, 2015…Dr. Helen Mayberg, a professor of psychiatry at Emory University, recently published a study in JAMA Psychiatry that identified a potential biomarker in the brain that could predict whether a depressed patient would respond better to psychotherapy or antidepressant medication.
Using PET scans, she randomized a group of depressed patients to either 12 weeks of treatment with the S.S.R.I. antidepressant Lexapro or to cognitive behavior therapy, which teaches patients to correct their negative and distorted thinking.
Over all, about 40 percent of the depressed subjects responded to either treatment. But Dr. Mayberg found striking brain differences between patients who did well with Lexapro compared with cognitive behavior therapy, and vice versa. Patients who had low activity in a brain region called the anterior insula measured before treatment responded quite well to C.B.T. but poorly to Lexapro; conversely, those with high activity in this region had an excellent response to Lexapro, but did poorly with C.B.T.
What might explain these different responses?
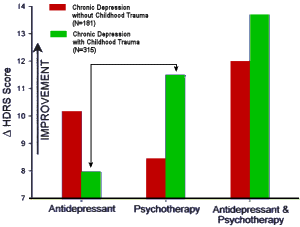
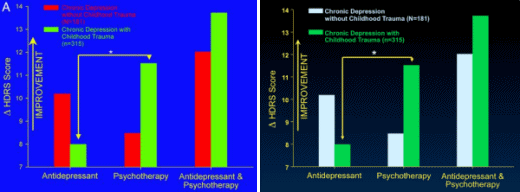
It turns out that other clinical factors may also help patients get the best treatment. For example, there is intriguing evidence that depressed patients who have a history of childhood trauma, such as the early loss of a parent or sexual or physical abuse, do not respond as well to an antidepressant as they do to psychotherapy.
In a large, multicenter study, Dr. Charles Nemeroff, then a professor of psychiatry at Emory and now at the University of Miami, found that for depressed adults without a history of abuse, there was a clear ranking order of treatment efficacy: Combined psychotherapy [using a form of cognitive behavior therapy] and an antidepressant [in this case, Serzone] was superior to either treatment alone. But for those who had a history of childhood trauma, the results were strikingly different: 48 percent of these patients achieved remission with psychotherapy alone, but only 33 percent of these patients responded to an antidepressant alone. The combination of psychotherapy and a drug was not significantly better than psychotherapy alone…
Erratum inProc Natl Acad Sci U S A. 2005 Nov 8;102(45):16530.
Erratum in Proceedings of the National Academy of Science. 2005 102[45):16530.
MEDICAL SCIENCES. For the article "Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma," which appeared in the Proc. Natl. Acad. Sci. in November 13, 2003, the authors note the following. "Results of the analyses of variance comparing change in Hamilton Rating Scale for Depression scores as a function of treatment type and early life trauma histories as well as Fig. 1A reflect change relative to the first week of treatment instead of baseline. When change scores relative to baseline are used, the interaction effects between treatment type and childhood trauma histories are not statistically significant…
This discrepancy is due to marked changes in depression scores during the first week of treatment. Note that all analyses comparing the more conservative outcome measure of remission as a function of treatment type and childhood trauma as well as Fig. 1B are correct. Thus, consideration of treatment response relative to baseline does not detect the effect of childhood trauma on final remission, whereas consideration of final response relative to first response does detect the effect."
2003 2012
by Martin B. Keller, M.D., James P. McCullough, Ph.D., Daniel N. Klein, Ph.D., Bruce Arnow, Ph.D., David L. Dunner, M.D., Alan J. Gelenberg, M.D., John C. Markowitz, M.D., Charles B. Nemeroff, M.D., Ph.D., James M. Russell, M.D., Michael E. Thase, M.D., Madhukar H. Trivedi, M.D., Janice A. Blalock, Ph.D., Frances E. Borian, R.N., Darlene N. Jody, M.D., Charles DeBattista, D.M.H., M.D., Lorrin M. Koran, M.D., Alan F. Schatzberg, M.D., Jan Fawcett, M.D., Robert M.A. Hirschfeld, M.D., Gabor Keitner, M.D., Ivan Miller, Ph.D., James H. Kocsis, M.D., Susan G. Kornstein, M.D., Rachel Manber, Ph.D., Philip T. Ninan, M.D., Barbara Rothbaum, Ph.D., A. John Rush, M.D., Dina Vivian, Ph.D., and John Zajecka, M.D.New England Journal of Medicine. 2000 342[20]:1462-1470.
…Conclusions: Although about half of patients with chronic forms of major depression have a response to short-term treatment with either nefazodone or a cognitive behavioral-analysis system of psychotherapy, the combination of the two is significantly more efficacious than either treatment alone.
by MARCIA ANGELL, MDNew England Journal of Medicine. 342[20]:1516-1518.In 1984 the Journal became the first of the major medical journals to require authors of original research articles to disclose any financial ties with companies that make products discussed in papers submitted to us. We were aware that such ties were becoming fairly common, and we thought it reasonable to disclose them to readers. Although we came to this issue early, no one could have foreseen at the time just how ubiquitous and manifold such financial associations would become. The article by Keller et al. in this issue of the Journal provides a striking example. The authors’ ties with companies that make antidepressant drugs were so extensive that it would have used too much space to disclose them fully in the Journal. We decided merely to summarize them and to provide the details on our Web site.
Finding an editorialist to write about the article presented another problem. Our conflict-of-interest policy for editorialists, established in 1990, is stricter than that for authors of original research papers. Since editorialists do not provide data, but instead selectively review the literature and offer their judgments, we require that they have no important financial ties to companies that make products related to the issues they discuss. We do not believe disclosure is enough to deal with the problem of possible bias. This policy is analogous to the requirement that judges recuse themselves from hearing cases if they have financial ties to a litigant. Just as a judge’s disclosure would not be sufficiently reassuring to the other side in a court case, so we believe that a policy of caveat emptor is not enough for readers who depend on the opinion of editorialists.
But as we spoke with research psychiatrists about writing an editorial on the treatment of depression, we found very few who did not have financial ties to drug companies that make antidepressants. [Fortunately, Dr. Jan Scott, who is eminently qualified to write the editorial, met our standards with respect to conflicts of interest.] The problem is by no means unique to psychiatry. We routinely encounter similar difficulties in finding editorialists in other specialties, particularly those that involve the heavy use of expensive drugs and devices…
And the end of all our exploring
Will be to arrive where we started
And know the place for the first time…
I questioned a doctor about their prescribing habits and was told bluntly that when I did a study, like the drug company, then I could comment on a certain drug. My reality is that when a new drug is announced there is a lead/lag in information. The drug company knows the only literature will be what they publish and it will take years for a proper meta analysis.
This gives them an opportunity to sell the drug, or device, and try to make it part of the standard of care for that issue. Drug companies are use to working with very long time horizons so a retraction years down the road will be met with silence in the hope that some of the literature will survive giving life to a discredited product.
Today we can look at the conflicts of various authors and question their bias in any new studies, remembering there is a pipeline of new KOL’s waiting in the wings. We can also look at the studies themselves and see the language of ghostwriting and that science has been kicked to the curb in favor of marketing.
Old studies are important since they are the only ones we can analyze and then use as a base line for behavior and truthfulness in the newer material.
A different doctor told me that you get what you pay for, so they relied on drug reps and journal articles for their prescribing information.
Fool me once….
Steve Lucas
Here is an example of how a simple idea can make it into common practice:
http://www.forbes.com/sites/larryhusten/2015/01/12/millions-of-americans-taking-aspirin-when-they-shouldnt/
Steve Lucas
Thanks so much for the perspective on the Nemeroff/Serzone article. I’d commented on the Friedman piece as I thought it was poor journalism in general–saying we’d be using imaging “soon” to direct antidepressant tx. (I’m a psychiatrist & nuc med doc, and had done PET research).
The study reported on is a research study & it’s a huge jump to think that it’s anywhere near clinical usefulness–For one thing 82 randomized patients but data used from only 38 of them (??!!). And we’re talking about activation in the insula on one side of the brain. What happened in Dr Mayberg’s former favorite–area 25 in the cingulate–oh, nothing : (
And then of all studies to pick to provide perspective–Nemeroff and say he was still at Emory, when the NYT had reported some of his stomach-turning “misadventures.”
Anyway, I’d like to bring to attention of Health News Review and cite 1BOM’s research if OK
Susan
Susan
Be my guest, and thanks for the comment.
Mickey
Susan Molchan is right to point out the inconsistencies and overstatements from the Mayberg group at Emory. A pointedly critical letter appeared in JAMA Psychiatry in response to the 2013 Mayberg report that Richard Friedman discussed. That link will give a further link to the full text. As well, in 2014 Mayberg published a somewhat confusing second report on the predictors of response in the same 82 patients. In that follow-up study there was no positive finding for the anterior insula which had been highlighted in the earlier study. In both studies there is reason to ask whether the authors were not HARKing – at the very least they were analyzing data selectively, as Dr. Molchan points out. We don’t know whether Helen Mayberg talked with Richard Friedman but if she did then she doesn’t seem to have mentioned the follow-up study.
Beware the Principal Investigator promoting a narrative in The New York Times and on the Charlie Rose show!
As twisted as all of this is, the conclusions of the NYT article does sort of comport to clinical experience and common sense.
Pretty pictures from brain scans — Phrenology anyone?
Hi Mickey–I got a blog posted on Health News Watch Dog re the Friedman NYT piece–you will enjoy, as will your readers, esp Dr. Carroll ; ) Thanks to both of you for your comments. Love your blog
http://www.healthnewsreview.org/2015/01/potential-biomarker-that-could-predict-caveats-about-psychiatric-brain-imaging-blogging-about-it/
Susan