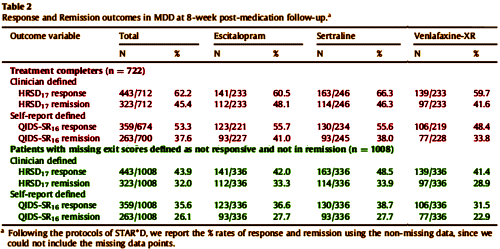
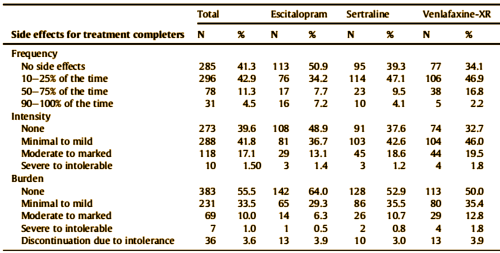
by Saveanu R, Etkin A, Duchemin AM, Goldstein-Piekarski A, Gyurak A, Debattista C, Schatzberg AF, Sood S, Day CV, Palmer DM, Rekshan WR, Gordon E, Rush AJ, Williams LM.Journal of Psychiatric Research. 2015 61:1-12.We aimed to characterize a large international cohort of outpatients with MDD within a practical trial design, in order to identify clinically useful predictors of outcomes with three common antidepressant medications in acute-phase treatment of major depressive disorder [MDD]. The international Study to Predict Optimized Treatment in Depression has presently enrolled 1008 treatment-seeking outpatients [18 – 65 years old] at 17 sites [five countries]. At pre-treatment, we characterized participants by symptoms, clinical history, functional status and comorbidity. Participants were randomized to receive escitalopram, sertraline or venlafaxine-extended release and managed by their physician following usual treatment practices. Symptoms, function, quality of life, and side-effect outcomes were assessed 8 weeks later. The relationship of anxiety to response and remission was assessed by comorbid Axis I diagnosis, presence/absence of anxiety symptoms, and dimensionally by anxiety symptom severity. The sample had moderate-to-severe symptoms, but substantial comorbidity and functional impairment. Of completers at week 8, 62.2% responded and 45.4% reached remission on the 17-item Hamilton Rating Scale for Depression; 53.3% and 37.6%, respectively on the 16-item Quick Inventory of Depressive Symptoms. Functional improvements were seen across all domains. Most participants had side effects that occurred with a frequency of 25% or less and were reported as being in the “none” to minimal/mild range for intensity and burden.
Outcomes did not differ across medication groups. More severe anxiety symptoms at pre-treatment were associated with lower remission rates across all medications, independent of depressive severity, diagnostic comorbidity or side effects. Across medications, we found consistent and similar improvements in symptoms and function, and a dimensional prognostic effect of comorbid anxiety symptoms. These equivalent outcomes across treatments lay the foundation for identifying potential neurobiological and genetic predictors of treatment outcome in this sample.
First, to state the obvious, this is a commercial study funded by Brain Resources. The authors in the by-line in red have received research support from Brain Resources. The ones in blue are employees of or principals in Brain Resources. And the underlined authors are the editor-in-chief and assistant editor of this very journal [Journal of Psychiatric Research]. So at a time when questions of conflict of interest have finally made it to the front page in medical research, these people don’t seem to have gotten the message. There are other anachronisms. Listed author AJ Rush was Director of TMAP and Principle Investigator for STAR*D and CO-MED – studies that notoriously churned out a seemingly endless stream of published papers. This article is, at best, an interim report, likely signalling yet another flood of articles pouring out of this iSPOT enterprise. And medical writer Jon Kilner who has been ghost?/editorial assistant? throughout the studies mentioned in the last post [a cul de sac I…], is still around doing whatever he does once more in this publication.
… iSPOT-D follows current usual care setting clinical practice in the prescription of antidepressant medications, coupled with collection of a broad range of potential predictor measures [e.g. genetics, neurobiological, psychophysiological etc]. This was done in order to arrive at a battery of tests that can be used in future prospectively-designed validation studies that will test the proposed individual patient-level treatment optimization algorithm built on these predictors.
 So here we are three decades after the first SSRI, two decades beyond the hypothesis that there’s some special way these drugs can be used to increase their efficacy looking at a study that says that they’re all the same. And we know what’s coming – a string of studies sifting through all tests neuro looking for something that will point to one or the other drug [with no real rationale that such a test might exist]. We have here a study financed by a commercial firm aiming for a future commercial product, authored by its employees, and published in a journal edited by some of the authors who are also involved with the company. The ticket into a peer reviewed medical journal is presumably the academic credentials of some of those authors.
So here we are three decades after the first SSRI, two decades beyond the hypothesis that there’s some special way these drugs can be used to increase their efficacy looking at a study that says that they’re all the same. And we know what’s coming – a string of studies sifting through all tests neuro looking for something that will point to one or the other drug [with no real rationale that such a test might exist]. We have here a study financed by a commercial firm aiming for a future commercial product, authored by its employees, and published in a journal edited by some of the authors who are also involved with the company. The ticket into a peer reviewed medical journal is presumably the academic credentials of some of those authors.
As I’ve said before, Alan Schatzberg is the Zelig of psychiatric malfeasance. How is it he’s everywhere there’s a moneybags involved?
It seems very likely that they are wasting their time and money chasing predictors of response in generic MDD. One just wishes that they wouldn’t waste our time as well with this soft-shoe product placement infomercial exercise designed to keep eyeballs on Brain Dynamics Centre and Brain Resource Company Operations Pty Ltd.
On checking the trial’s registration at ClinicalTrials.gov, some questions arise.
1. This new report comprises 1,008 patients, though they say their goal is to recruit 2,688 patients. However, only 2 of the 22 listed study sites are said to be currently recruiting. How come? Moreover, there was a 28% dropout rate, so that only 722 patients completed 8 weeks of treatment. With such a long way to go, why rush to publish this interim report? For PR reasons it would seem.
2. They have reported no results on ClinicalTrials.gov even though this new report was submitted on 3 March, 2014. The last revision of the information on ClinicalTrials.gov was 6 months ago – 18 August, 2014. By now they must have had over a year since data analyses were completed to get the information posted. There seems to be no sense of urgency on their part.