NASDAQby Zacks Equity ResearchJune 13, 2012
… This will be the catalyst to driving growth of the personalized medicine business, Brain Resource’s major focus and the area that is expected to be the impetus to the company’s long-term revenue and earnings growth. Findings from the iSPOT trial will be used to develop these depression and ADHD biomarkers. iSPOT is the world’s largest clinical trial to predict treatment response in depression and ADHD. iSPOT-D [for depression] has enrolled over 1,700 patients and analysis started on the first 1,000 patients. Data from iSPOT-D was presented at two major U.S. medical conferences during 2011, including an invitation-only presentation at The American Conference of Neuropsychopharmacology in December and more recently formed a panel of presentations at NCDEU in Arizona… Brain Resource is planning to submit study outcomes to the FDA for approval [likely via PMA] of a depression and an ADHD test in the near-to-mid term [discussions with the FDA regarding the regulatory approval pathway are ongoing]…
iSPOT-D “Test” cohort (n=1008) complete. First “Replication” cohort (n=700) is locked.FDA meeting on first biomarker outcomes from the iSPOT studies. View Report
3 July 2012FDA meeting on BRC’s Depression Treatment TestBRC met with the FDA [June 28] t o discuss our Pre-IDE submission in regards to the company’s test to predict optimized treatment for D epression based on our international iSPOT study. This was a positive meeting which addressed the issues previously raised by the FDA. Significantly, the FDA considered a de novo pathway as a possible pathway for our submission. The implication of this being typically less complexity and shorter approval times as compared to a PMA submission . As such, we continue to believe we are on track to develop and obtain FDA marketing clearance in the United States for a personalized predictive test in Depression. Our next steps include filing a Pre-IDE supplement to clarify the pivotal validation study [expected within the next two months], this laying the ground for us to begin working on our final submission.
iSPOT-D Publication Management TeamThe Publication Management Team [PMT] is the administrative body that provides support and structure to ensure a large volume of high caliber papers are produced from the data. The iSPOT-D PMT follows a structured publication plan and has been formed with John Rush, MD as chairman. John Rush, MD was the PI of the first-of-its’ kind STAR*D Depression research study that produced over 120 papers [published across more than 20 peer reviewed journals internationally].
-
"Dr. Williams has previously received fees as a consultant for Brain Resource Ltd and in the last 3 years and was a stockholder in Brain Resource Ltd."
-
"iSPOT-D is sponsored by Brain Resource Company Operations Pty Ltd. Dr Williams was the Academic Principal Investigator for iSPOT-D from 2008 to 2013."
-
Dr. Williams is now at Stanford working on the NIMH RDoC, iSPOT, and PTSD…
-
And speaking of a change, Dr. Charlie Nemeroff was a prominent member of the Brain Resources team. However, in the recent paper, first author Radu Saveanu, his Director of Education, represented the University of Miami instead [detoxifying the by-line?].
I haven’t a clue about the answer to any of these questions, but I do know this. This entire personalized medicine scenario has been heavily colored by a quest to find a marketable pick-the-antidepressant test. It has played out among the usual suspects of entrepreneurial psychiatry in high places, spread through the pages of academic peer-reviewed journals, and been nurtured in the departments of psychiatry at some prestigious universities. In fact, this whole story going back to TMAP could be folded into that last sentence – much ado about not very much. Haven’t we explored this cul de sac long enough?
heavily colored by a quest to find a marketable pick-the-antidepressant test. It has played out among the usual suspects of entrepreneurial psychiatry in high places, spread through the pages of academic peer-reviewed journals, and been nurtured in the departments of psychiatry at some prestigious universities. In fact, this whole story going back to TMAP could be folded into that last sentence – much ado about not very much. Haven’t we explored this cul de sac long enough?
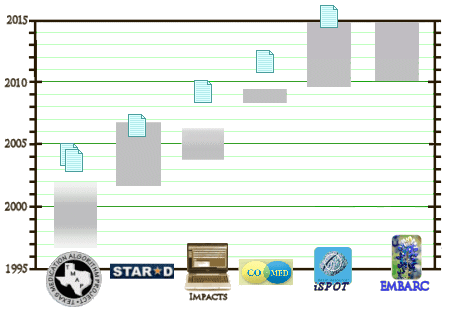
So, the plot thickens… Good sleuthing, Dr. Mickey.
According to what patients report on my site, psychiatrists often tell them that personalized drug treatment plans are on the way. Hope springs eternal….