

by Fleischhacker WW, Sanchez R, Perry PP, Jin N, Peters-Strickland T, Johnson BR, Baker RA, Eramo A, McQuade RD, Carson WH, Walling D, and Kane JM.British Journal of Psychiatry. 2014 205[2]:135-44.[clinicaltrials.gov: NCT00706654][with results]
The authors in red are either heavily industry-funded by or employees of Otsuka, The Study Director was Otsuka employee, Raymond Sanchez. There were 98 clinical trial sites, and an army of editorial assistants and consultants suggesting that it was ghost-written and ghost-analyzed. The senior listed author, John Kane, is in charge of the influential ACA funded NIMH RAISE study of the treatment of First Episode Schizophrenia. This study analysis seemed legit to me.
Except for the fact that the industry credits scrolled by like at the end of a Hollywood Blockbuster, and I kind of wonder why the industries were mostly for-hire or why they even bother to list academic authors as anything other than ticket holders for admission to an academic journal, my point really isn’t about Abilify Maintena® or even Long Acting Injectable antipsychotics. It’s about advertisement and conflicts of interest. Continuing Medical Education was made a requirement for licensure in my medical lifetime. The intent was to insure that as doctors age, they are kept abreast of the rapidly changing medical/scientific landscape. And our peer reviewed medical journals perform the same function. Traditionally, individual physicians paid for both – CME presentations and our Journals. But sometimes they are free…
So last time, we had an LAI paper from an author at the Rand Corporation on the Janssen payroll [impossibility…]. Now comes a Free CME that was "supported by an independent educational grant from Otsuka America Pharmaceuticals, Inc", who happen to have a new LAI on the market [Abilify Maintena®], approved by the FDA based on an industry study with senior author John Kane, who happens to be funded himself by Otsuka, who happens to direct an NIMH Project treating Schizophrenia being picked up already by SAMHSA’s Block Grant program, who happens to write about the drugs used to treat Schizophrenia, and who was skeptical about Tardive Dyskinesia as far back as 1982.
-
Christoph U. Correll, MD
Professor of Psychiatry and Molecular Medicine
Hofstra North Shore – LIJ School of MedicineMedical Director of Recognition and Prevention (RAP) Program
The Zucker Hillside Hospital
Glen Oaks, NY -
John M. Kane, MD
Professor of Psychiatry
Hofstra North Shore – LIJ Health System School of Medicine
Vice President
Behavioral Health Services of the North Shore – LIJ Health System
Chairman of Department of Psychiatry and Chief of Staff
The Zucker Hillside Hospital
Glen Oaks, New York -
John Lauriello, MD
Chancellor’s Chair of Excellence in Psychiatry
Executive Medical Director of the Missouri Psychiatric Center
School of Medicine, University of Missouri Health System
Columbia, MO
Take a look at the Learning Objective and guess what you’re going to learn – for free. Oh by the way, John Lauriello, MD is the senior author of a glowing handout review of Abilify Maintena®.
I think that the results are believable despite the conflict of interest. You can find the same results from other LAIs in the past. The problem is that the side effects of LAIs are always minimized compared to what is encountered in clinical practice and patient acceptance is maximized. We have had presentations on LAIs in the past. In clinical practice a minority of patients consent to this treatment after a neutral presentation. That is true even if the long acting injectable is not an antipsychotic drug (like long acting naltrexone). The other issue is risk of tardive dyskinesia. Per the graphic you previously posted TD risk with LAIs is higher.
While the results are plausible, that is not the problem in clinical practice. Side effects and patients acceptance are larger issues. It is also critical that the patient be seen frequently to determine the minimum effective dose prior to changing over to the LAI. How to convert that to the injectable is frequently not clear. The other issue for clinicians is that they can anticipate being harassed for prior authorization with any expensive (and in some cases inexpensive) AAP.
This really a thank you for doing this work. I am a retired university psychiatry professor who is 67. I have a small private practice now, and, yes do psychotherapy as well as the drugs. Everything you so comment upon with such such concision is the sort of thing I tried to teach the residents for many years. I only retired two years ago. Something very sinister had begun to happen. Residents were beginning to lose their curiosity about just about everything. They generally, with a few exceptions, just wanted to get the sheepskin and get out. I worked quite a lot with residents, am a good teacher with plenty of gratitude and awards (!), but it was becoming impossible to get them interested in anything, anything, beyond “getting through.” Ah temporah. Ah mores! Thank you again. John Neill
An article by Szfranski concerning olanzepine’s association with tardive dyskenisia stated that :
“The study demonstrated high (16,4%) prevalence of tardive dyskinesia, and the annual incidence (3,9%) comparable to the results of a meta-analysis by Corell et al. In the case of olanzapine in monotherapy the annual incidence was lower (1.96%) but the use of antipsychotics in polytherapy more than tripled the risk of tardive dyskinesia.”
Does this sound like your experience?
John,
You tell a sad story, but as Scarlett said, “Tomorrow is another day.” Today’s residents are entering a different world than we did, it’s hard to imagine. I still work some in a free clinic for the uninsured in a rural area, but it’s as rewarding as it always was. People haven’t changed, even if the systems around them rise and fall, come and go.
Sounds like you’ve had a good career. I did too…
Thanks for writing…
George,
I’ve wondered if having the steadier, constant blood levels with the Injectables as opposed to the pulsing of oral meds might have some impact on the neurological effects. Whatever the mechanism, it seems consistent that they are TD/EPS prone.
I’m thinking that the rash of LAIs is a patent extending mechanism much like the indication creep seen in the past.
Ferrell,
I don’t personally see many psychotic patients, and I never use Olanzapine. The incidence of weight gain and metabolic syndrome [diabetes] is unacceptable. The figures from that polish study seem high to me, but plausible. Tardive Dyskinesia in its full blown form isn’t common, but you only have to see it a few times to live in fear of causing it. Here‘s what a former drug rep had to say about the drug.
Global Academy for Medical Education, joint sponsor of the above CME, is a MECC — a Medical Education Communication Company. As discussed here, MECCs are “independent profit-based companies many of which have been set up by advertising/marketing agencies. MECCs were a convenient way of establishing a ‘firewall’ between the agency’s promotional side and its medical education side.”
To be fair, MECCs run the gamut: some are squeaky clean while others are pharma ad agencies by another name. My quick web investigation revealed no smoking gun connecting this company to a specific drug manufacturer. However, their Chief Strategist for Digital CME until two months ago was Stephen Smith, who formerly pioneered electronic pharmaceutical marketing. Incestuous relationships with pharma are likely the rule more than the exception in this industry. You said it well: even with every “i” dotted and “t” crossed, with a COI trail like this, we really can’t believe a word of it.
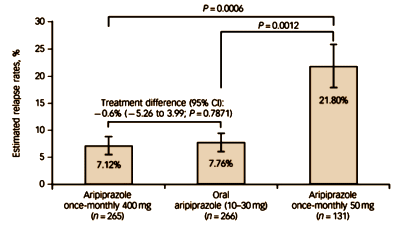
Maybe I am missing it but it does not look like the data above show and advantage for the LAI over the oral (and soon to be off patent?) form of the drug.
The part that is slippery is that they took patients stabilized on Abilify and abruptly transitioned them to one of the three arms. That seems to give an advantage to the group who remained on the oral form and the group randomized to the higher dose of the LAI.
Sandra,
It doesn’t show any advantage. It shows “non-inferiority” meaning something like “just as good as” as I understand it. What they’re implying is that the advantage is that the patients are guaranteed to be “on meds” – as in “compliant” or “adherent.” What we think is that it’s a patent extender, as in Paxil->PaxilCR or Celexa->Lexapro or Effexor->Pristiq or Risperdal -> Consta ->Sustenna – just tricks of the trade…