![]()
by Anderson ML, Chiswell K, Peterson ED, Tasneem A, Topping J, and Califf RMThe New England Journal of Medicine. 2015 372[11]:1031-1039.
BACKGROUND: The Food and Drug Administration Amendments Act [FDAAA] mandates timely reporting of results of applicable clinical trials to ClinicalTrials.gov. We characterized the proportion of applicable clinical trials with publicly available results and determined independent factors associated with the reporting of results.METHODS:Using an algorithm based on input from the National Library of Medicine, we identified trials that were likely to be subject to FDAAA provisions [highly likely applicable clinical trials, or HLACTs] from 2008 through 2013. We determined the proportion of HLACTs that reported results within the 12-month interval mandated by the FDAAA or at any time during the 5-year study period. We used regression models to examine characteristics associated with reporting at 12 months and throughout the 5-year study period.RESULTS: From all the trials at ClinicalTrials.gov, we identified 13,327 HLACTs that were terminated or completed from January 1, 2008, through August 31, 2012. Of these trials, 77.4% were classified as drug trials. A total of 36.9% of the trials were phase 2 studies, and 23.4% were phase 3 studies; 65.6% were funded by industry. Only 13.4% of trials reported summary results within 12 months after trial completion, whereas 38.3% reported results at any time up to September 27, 2013. Timely reporting was independently associated with factors such as FDA oversight, a later trial phase, and industry funding. A sample review suggested that 45% of industry-funded trials were not required to report results, as compared with 6% of trials funded by the National Institutes of Health [NIH] and 9% of trials that were funded by other government or academic institutions.CONCLUSIONS: Despite ethical and legal obligations to disclose findings promptly, most HLACTs did not report results to ClinicalTrials.gov in a timely fashion during the study period. Industry-funded trials adhered to legal obligations more often than did trials funded by the NIH or other government or academic institutions.[Funded by the Clinical Trials Transformation Initiative and the NIH.]
Just this week, I had an example of how useful the Results Database of ClinicalTrials.gov can be [see inertia…]. I was looking at the Clinical Trials for Saphris® [Asenapine] that was submitted for its FDA Approval [NCT01244815 and NCT01349907]. There’s no published paper, but I happened to notice that the Results of these Clinical Trials were already posted – a miracle all on its own! And they had something of an answer to an open question. In the first trial, they gave Saphris® for three weeks to kids diagnosed with a Manic episode:
| Acute: Measured Values after 3 Weeks
|
||||
| Placebo | Asenapine 2.5 mg BID |
Asenapine 5.0 mg BID |
Asenapine 10.0 mg BID |
|
| Participants | 79 | 88 | 87 | 81 |
| Change in Y-MRS Mean ± SD |
-9.6 ± 7.8 | -12.3 ± 9.0 | -15.1 ± 9.5 | -15.9 ± 9.1 |
| P Value | =0.008 | <0.001 | <0.001 | |
There’s no surprise here – that one can knock down an agitated state with an antipsychotic. But these drugs have been used long term in these patients. What about that? In the extension study, things didn’t look so rosy:
| Maintenance: After 50 Weeks of Asenapine
|
||
| Acute | Asenapine/Asenapine | Placebo/Asenapine |
| Participants | 241 | 80 |
| With Adverse Event | 197 | 74 |
| Completed Study | 102 | 38 |
I guess they were looking for a maintenance selling point in the extension phase of the study, but they sure didn’t find it – quite the opposite. One way to not deal with that finding would be to not publish it [publication bias]. Requiring a completed Results Database in ClinicalTrials.gov blocks that solution deception – instead it adds yet another piece of evidence that maintenance Atypical Antipsychotics are fraught with problems.
 the drugs I prescribe. But the pharmaceutical industry has systematically used them as ways to deceive me [us] – engaging physicians in high places to collude with them in the process. This may be only one of the holes in the system that keeps me up to date on medications, but it’s time to put a cork in it and move on to the next leaky spot in the dyke…
the drugs I prescribe. But the pharmaceutical industry has systematically used them as ways to deceive me [us] – engaging physicians in high places to collude with them in the process. This may be only one of the holes in the system that keeps me up to date on medications, but it’s time to put a cork in it and move on to the next leaky spot in the dyke…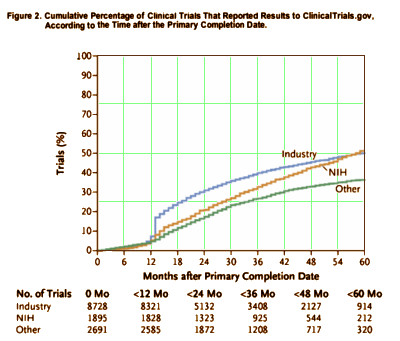
JL
If you see this-
I missed an opportunity to comment yesterday. I appreciate reading your contributions to this important discussion as I have in the past.
JL
As Sandra Steingard I appreciate your comments, and hope to be able to read more from you, on this most excellent blog by dr Nardo, which, ever so often, is marred by arrogance and incivility, not by dr Nardo, but by less sensitive men. They can be skipped. I usually do. Au revoir!
Ditto.