Lundbeck Press ReleaseSeptember 24, 2014
H. Lundbeck A/S [Lundbeck] and Otsuka Pharmaceutical Co., Ltd. [Otsuka] today announced that the US Food and Drug Administration [FDA] has determined that the New Drug Application [NDA] for brexpiprazole for monotherapy in adult patients with schizophrenia and for adjunctive treatment of major depressive disorder [MDD] in adult patients is sufficiently complete to allow for a substantive review and the NDA is considered filed as of 9 September 2014 [60 days after submission]. The PDUFA date is July 11, 2015…
PsychiatricNewsApril 17, 2015New findings from a phase 3 clinical trial, published today in AJP in Advance, suggest that a recently developed antipsychotic may prove to be one of the next treatments for schizophrenia. Researchers from the Department of Psychiatry at Hofstra North Shore-LIJ School of Medicine conducted a randomized, double-blind, placebo-controlled study with 636 patients with schizophrenia to investigate the efficacy, safety, and tolerability of brexpiprazole—a serotonin-dopamine activity modulator that acts as a partial agonist at serotonin 5-HT1A receptors and dopamine D2 receptors, while antagonizing serotonin 5-HT2A receptors and noradrenaline alpha receptors…
“It is important for clinicians and patients to have a range of treatment options to manage symptoms effectively and safely … as response to therapy can vary greatly from individual to individual and from one medication to the next.” Correll informed Psychiatric News that the Food and Drug Administration will make its final decision about the approval of brexpiprazole for the treatment of schizophrenia as well as major depressive disorder in July…

by Correll CU, Skuban A, Ouyang J, Hobart M, Pfister S, McQuade RD, Nyilas M, Carson WH, Sanchez R, and Eriksson H.American Journal of Psychiatry. 2015 Apr 16 [Epub ahead of print]
OBJECTIVE: The efficacy, safety, and tolerability of brexpiprazole and placebo were compared in adults with acute schizophrenia.METHOD: This was a multicenter, randomized, double-blind, placebo-controlled study. Patients with schizophrenia experiencing an acute exacerbation were randomly assigned to daily brexpiprazole at a dosage of 0.25, 2, or 4 mg or placebo [1:2:2:2] for 6 weeks. Outcomes included change from baseline to week 6 in Positive and Negative Syndrome Scale [PANSS] total score [primary endpoint measure], Clinical Global Impressions Scale [CGI] severity score [key secondary endpoint measure], and other efficacy and tolerability measures.RESULTS: The baseline overall mean PANSS total score was 95.2, and the CGI severity score was 4.9. Study completion rates were 62.2%, 68.1%, and 67.2% for patients in the 0.25-, 2-, and 4-mg brexpiprazole groups, respectively, versus 59.2% in the placebo group. At week 6, compared with placebo, brexpiprazole dosages of 2 and 4 mg produced statistically significantly greater reductions in PANSS total score [treatment differences: -8.72 and -7.64, respectively] and CGI severity score [treatment differences: -0.33 and -0.38]. The most common treatment-emergent adverse event for brexpiprazole was akathisia [2 mg: 4.4%; 4 mg: 7.2%; placebo: 2.2%]. Weight gain with brexpiprazole was moderate [1.45 and 1.28 kg for 2 and 4 mg, respectively, versus 0.42 kg for placebo at week 6]. There were no clinically or statistically significant changes from baseline in lipid and glucose levels and extrapyramidal symptom ratings.CONCLUSIONS: Brexpiprazole at dosages of 2 and 4 mg/day demonstrated statistically significant efficacy compared with placebo and good tolerability for patients with an acute schizophrenia exacerbation.
Dr. Correll has been a consultant and/or advisor to or has received honoraria from Actelion, Alexza, American Academy of Child and Adolescent Psychiatry, Bristol-Myers Squibb, Cephalon, Eli Lilly, Genentech, Gerson Lehrman Group, IntraCellular Therapies, Lundbeck, Medavante, Medscape, Merck, National Institute of Mental Health, Janssen/J&J, Otsuka, Pfizer, ProPhase, Roche, Sunovion, Takeda, Teva, and Vanda; he has received grant support from Bristol-Myers Squibb, Feinstein Institute for Medical Research, Janssen/J&J, National Institute of Mental Health, NARSAD, and Otsuka; and he has been a Data Safety Monitoring Board member for Cephalon, Eli Lilly, Janssen, Lundbeck, Pfizer, Takeda, and Teva.
Funded by Otsuka Pharmaceutical Development & Commercialization, Inc., and H. Lundbeck A/S. Jennifer Stewart, M.Sc. [QXV Communications, Maccles field, U.K.] provided writing support that was funded by Otsuka Pharmaceutical Development & Commercialization, Inc., and H. Lundbeck A/S.
by Kane JM, Skuban, Ouyang, Hobart, Pfister, McQuade, Nyilas, Carson, Sanchez, and Eriksson.Schizophrenia Research. 2015 Feb 12. [Epub ahead of print]
The objective of this study was to evaluate the efficacy, safety and tolerability of brexpiprazole versus placebo in adults with acute schizophrenia. This was a 6-week, multicenter, placebo-controlled double-blind phase 3 study. Patients with acute schizophrenia were randomized to brexpiprazole 1, 2 or 4mg, or placebo [2:3:3:3] once daily. The primary endpoint was changed from baseline at week 6 in Positive and Negative Syndrome Scale [PANSS] total score; the key secondary endpoint was Clinical Global Impressions-Severity [CGI-S] at week 6. Brexpiprazole 4mg showed statistically significant improvement versus placebo [treatment difference: -6.47, p=0.0022] for the primary endpoint. Improvement compared with placebo was also seen for the key secondary endpoint [treatment difference: -0.38, p=0.0015], and on multiple secondary efficacy outcomes. Brexpiprazole 1 and 2mg also showed numerical improvements versus placebo, although p>0.05. The most common treatment-emergent adverse events were headache, insomnia and agitation; incidences of akathisia were lower in the brexpiprazole treatment groups [4.2%-6.5%] versus placebo [7.1%]. Brexpiprazole treatment was associated with moderate weight gain at week 6 [1.23-1.89kg versus 0.35kg for placebo]; there were no clinically relevant changes in laboratory parameters and vital signs. In conclusion, brexpiprazole 4mg is an efficacious and well-tolerated treatment for acute schizophrenia in adults… BEACON trial.
[recolored to match the graph above]
Dr Kane has been a consultant for Amgen, Alkermes, Bristol-Meyers Squibb, Eli Lilly, EnVivo Pharmaceuticals [Forum] Genentech, H. Lundbeck. Intracellular Therapeutics, Janssen Pharmaceutica, Johnson and Johnson, Merck, Novartis, Otsuka, Pierre Fabre, Proteus, Reviva, Roche and Sunovion. Dr Kane has been on the Speakers Bureaus for Bristol-Meyers Squibb, Eli Lilly, Janssen, Genentech and Otsuka, and is a shareholder in MedAvante, Inc.
Ruth Steer, PhD, [QXV Communications, Macclesfield, UK] provided writing support, which was funded by Otsuka Pharmaceutical Development & Commercialization, Inc. [Princeton, USA] and H. Lundbeck A/S [Valby, Denmark].
From the Zucker Hillside Hospital, Glen Oaks, N.Y.; Otsuka Pharmaceutical Development & Commercialization, Princeton, N.J.; and H. Lundbeck A/S, Valby, Copenhagen, Denmark.
I just wrote a three part series on the Academic·Industrial·Complex that was as overly detailed as this post – which is yet another example of what that phrase means. I think I’ve been chasing down the details, looking in vain for something that breaks out of the mold of scientific enterprise being used as a commercially driven advertising platform. And what I find is that the further I look, the worse it gets. I was tempted to say that Brexpiprazole is a weak sister Atypical Antipsychotic with a low Adverse Event profile, but I’m not even sure that’s defensible with just two six week trials. And unmentioned here, they used some idiosyncratic analytic techniques that were unfamiliar to me, but I just didn’t have the libido to look into them further [because there was no primary data to test them with].
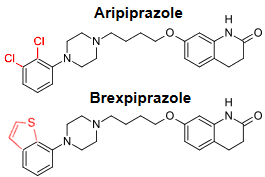
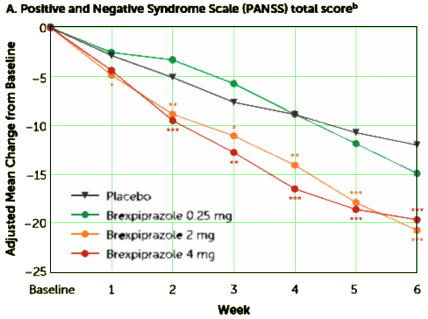
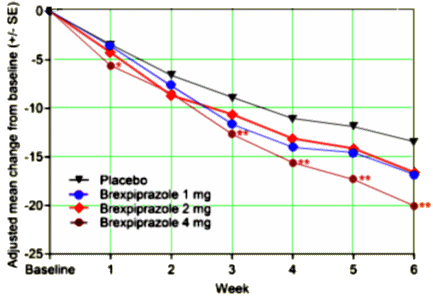
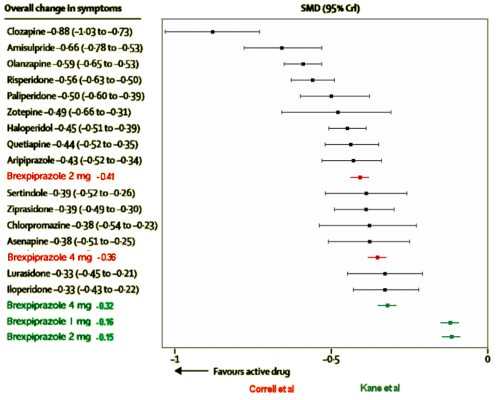
To repeat: the results are of no importance beyond the corporate narrative. The corporate owners tested this new drug against their blockbuster drug aripiprazole in only one of the 30 studies listed on ClinicalTrials.gov. No results on display, yet, as Dr. Mickey pointed out. Plus, they tested their new drug only once against any other approved antipsychotic drug – that was quetiapine. So much for the sanctimonious PR talk about how, quoting Dr. Correll, “It is important for clinicians and patients to have a range of treatment options to manage symptoms effectively and safely …” Absent comparative trials, how could one tell whether brexpiprazole is more than an expensive me-too drug or even maybe not as good as aripiprazole? It’s time for the FDA to get serious about comparative efficacy trials.
As always, thanks for taking the time to write this. And certainly not too long.
There are a few things patients and those in medicine need to remember:
Business people are dumb. They will continue to do the same thing over and over as long as it produces results. So we see the same behavior of using researches in name only and ghostwriters as we have seen in the past.
On the psychological front every sales person, and those in sales organizations, feels they are smarter than the guy who got caught. Here we see an attempt to bury the researchers and ghostwriters conflicts. Nobody will notice if we place this information in two different trials since you would have to be a Dr. John Nardo to check.
There is only one rule in managing sales people:
“Facts are the enemy of truth.”
Sales people by their nature are charming, tuned into you feelings and often lie. While they use to be held in check by managers today they are the top performers in many organizations. Snakes in Suits does a good job of describing this nonviolent psychopath at work. Their focus is only on themselves, with a win at any cost mentality that results in chaos in the work place, and often a troubling personal life.
The goal, as Dr. Carroll points out, is the corporate narrative and all the good corporate soldiers will fall in line and maybe if this thing takes off there will be a raise. Money is how we keep score and if my product sells more than yours, or I make more than you, I win. The effectiveness of the product is not important.
Steve Lucas
Research as sales and marketing is appalling, but not all sales and marketing people would approve of this kind of thing.
I knew someone who marketed latex gloves and suture supplies and then sold medical supplies to distributors who sold to doctors (in Canada). He’s not the smartest person I’ve ever met, but he wasn’t dumb. He knew how his products worked, and he took care of his customers by not selling them more than they needed and buying back excess. He saw himself as having a long-term relationship with the people who used his products. He would not approve of these practices. My point is that even sales and marketing people can have standards for ethical behavior.
I
When J&J had problem with Tylenol in the 80’s they were open about it and acted, because the business people thought that that was important for their reputation. Contrast that with the recent quality problems with children’s Tylenol.
What the hell, another dog and pony show, for a year promoting the need for yet another antipsychotic drug that will then become the next “mood stablilizer”, then adjunct antidepressant, and who knows, maybe the next cure for cancer?!
Perhaps, though, the door to open to who are the real criminal element in psychiatry promoting yet another profit driven agenda without appropriate vetting and justification to be considered first.
Yeah, there is the real PR machine at work by colleagues who are doing what is right and responsible. But, don’t hold your breath.
Unless you are about to die!
Harsh comment, nah, watching the antisocial crap socially and politically can lead to much worse. And much justified.
Check out the near rioting going on in my town, Brawl-timore! Incredible what passes as protesting. Kinda like what antipsychiatry thinks they can pass by readers at sites like this.
Sorry for the digressing. Reading this post is just another example of why people are just numb from the dumbing down of responsibility.
Hey, to those readers wondering why so many psychiatrists buy into this crap with big pharma, why don’t you ask yourself why an entrenched 35% plus of the electorate support Hillary Clinton even with all the growing revelations of frank criminal, corrupt behaviors while she was Secretary of State. Agendas trump principles pretty damn easily these days.
End of line.
Not two long. You’re time that you put into these “investigative journalism ” I very much appreciate. Have you ever thought about starting a newsletter a la Carlat? I think you’d have enough subscribers to cover costs, even make a profit, Hire out the printing & distribution. Worth a try? In my view you are hiding your lamp under a bushel now. JN