Dr. Keller contacted The Chronicle on Wednesday to insist that the 2001 results faithfully represented the best effort of the authors at the time, and that any misrepresentation of his article to help sell Paxil was the responsibility of Glaxo. "Nothing was ever pinned on any of us," despite various trials and investigations, he said. "And when I say that, I’m not telling you we’re like the great escape artists, that we’re Houdinis and we did something wrong and we got away with the crime of the century. Don’t you think if there was really something wrong, some university or agency or something would have pinned something on us?" In what he described as his first effort to speak publicly about the matter, Dr. Keller said his critics also have financial and professional motives for amplifying criticisms, including lawyers representing Paxil plaintiffs and professors seeking their own records of journal publication…
“The group is a self-appointed watchdog,” Jeffrey Lieberman, chair of psychiatry at the Columbia University College of Physicians and Surgeons, told BuzzFeed News. “One wonders what the motivation is, and how objective they’re going to be.”
“The study authors comprised virtually all of the academic researchers studying the treatment of child depression in North America at the time”…
MARTIN B. KELLER, M.D., NEAL D. RYAN, M.D., MICHAEL STROBER, PH.D., RACHEL G. KLEIN, PH.D., STAN P. KUTCHER, M.D., BORIS BIRMAHER, M.D., OWEN R. HAGINO, M.D., HAROLD KOPLEWICZ, M.D., GABRIELLE A. CARLSON, M.D., GREGORY N. CLARKE, PH.D., GRAHAM J. EMSLIE, M.D., DAVID FEINBERG, M.D., BARBARA GELLER, M.D., VIVEK KUSUMAKAR, M.D., GEORGE PAPATHEODOROU, M.D., WILLIAM H. SACK, M.D., MICHAEL SWEENEY, PH.D., KAREN DINEEN WAGNER, M.D., PH.D., ELIZABETH B. WELLER, M.D., NANCY C. WINTERS, M.D., ROSEMARY OAKES, M.S., AND JAMES P. MCCAFFERTY, B.S.
ANSWER: Yes
QUESTION: Is this a document that you were referring to that you got the data from?
ANSWER: I don t recall what specific document I did receive Whether it was this was one. I mean yes this would be what I would have gotten. I don’t recall getting it.
QUESTION: You don t recall ever receiving it but you know you got it right?
ANSWER: Yes I got it. Yes I don’t recall receiving it.
QUESTION: This provided you with information that you then utilized to prepare the first draft of the manuscript for Study 329?
ANSWER: Yes
ANSWER: I believe I created it on my own.
QUESTION: Did Martin Keller tell you what to put in the first draft?
ANSWER: I don’t recall. I don t think I had any conversation with him until we were, you know, afterwards.
QUESTION: After you prepared the first manuscript?
ANSWER: To the best of my recollection, yes.
ANSWER: Yes
QUESTION: And you can’t tell from reading – a reader could not distinguish which are these – whether any or all of them are primary or secondary?
ANSWER: Correct
QUESTION: My question was do you know whose idea it was to not distinguish between primary and secondary efficacy measures?
ANSWER: A reader cannot. This was a first draft, so this came straight from me. This was, I guess, my interpretation. I’m remembering this may have been my interpretation of the data.
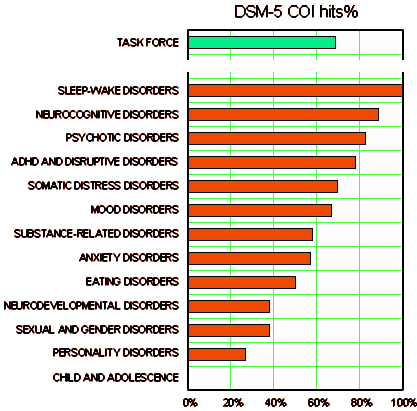 Similar experts with [financial] PHARMA COI are everywhere: CME presentations; Speaker’s Bureaus; Review Articles; the list gets longer by the year. A recent NEJM series [wtf?…, wtf? for real…] argued that their longstanding policy of banning authors with these COI from review articles should change [in part because there’s a paucity of "clean" experts]. The DSM-5 Revision was done using panels of experts heavily laden with COI tainted members [must be crazy…]. Expert "panels" produced the guidelines and algorithms for the infamous TMAP scam in Texas [1999…]. It appears that we have developed a "cult" of experts [called Key Opinion Leaders by the pharmaceutical marketers].
Similar experts with [financial] PHARMA COI are everywhere: CME presentations; Speaker’s Bureaus; Review Articles; the list gets longer by the year. A recent NEJM series [wtf?…, wtf? for real…] argued that their longstanding policy of banning authors with these COI from review articles should change [in part because there’s a paucity of "clean" experts]. The DSM-5 Revision was done using panels of experts heavily laden with COI tainted members [must be crazy…]. Expert "panels" produced the guidelines and algorithms for the infamous TMAP scam in Texas [1999…]. It appears that we have developed a "cult" of experts [called Key Opinion Leaders by the pharmaceutical marketers].
-
If there is evidence that a person is biased in some manner that would affect the reliability of her claims, then an Argument from Authority based on that person is likely to be fallacious. Even if the claim is actually true, the fact that the expert is biased weakens the argument. This is because there would be reason to believe that the expert might not be making the claim because he has carefully considered it using his expertise. Rather, there would be reason to believe that the claim is being made because of the expert’s bias or prejudice.
-
If a person makes a claim about some subject outside of his area(s) of expertise, then the person is not an expert in that context. Hence, the claim in question is not backed by the required degree of expertise and is not reliable. It is very important to remember that because of the vast scope of human knowledge and skill it is simply not possible for one person to be an expert on everything. Hence, experts will only be true experts in respect to certain subject areas. In most other areas they will have little or no expertise.
I actually sometimes feel sorry for some of the people on that BY·LINE. For some, I expect their error was in assuming that the analysis of the study was properly conducted. But my empathy is short-lived. I think they must’ve felt like it was a double return for their efforts. By doing the study on their site, they raised money for their departments AND they added an article to their respective resumes. What they got was anything but a boost to their status as experts. They delegated their author·ity to other experts who were operating deep in the domain of fallacy – then compounded and continue to compound the problem by their silence.
… As with most scientific papers, Keller and colleagues convey an impression that “the data have spoken.” This authoritative stance is possible only in the absence of access to the data. When the data become accessible to others, it becomes clear that scientific authorship is provisional rather than authoritative.
Too bad it cannot be known whether what Keller said about the timing of inclusion of the secondary measures (before or after blinding) is true or not. It seems like there’s some chance that he is lying to protect himself, although one cannot accuse him of that without evidence.
Edward,
True. But changing the outcome variable as the study winds up at the end isn’t kosher no matter what.
Valuable as the current retrospective on Study 329 is — and I count it as very valuable indeed — we need to heed the wisdom of Pharma executive, Leigh Thompson, advising his troops, in 1991, on where the struggle is lost and won. At the time, he was defending Prozac:
“When you battle the media and politicians, the only thing that counts is the first word. The rebuttals are always on the last page and forgotten.” (Quoted in D. Healy blog post, 9.21.15, “What Happened to those Suicidal in Study 329?”)
Jeffrey Lieberman’s comment is interesting, Here is a physician who clearly cannot accept that any group would want to correct the record in the public interest. One of the most interesting revelations of this whole 329 journey has been how poorly highly educated people understand CONFLICT OF INTEREST..
Julie,
Highly educated people now consider conflicts of interest to be SYNERGIES…
I didn’t come up with that quote, this guy did:
https://en.wikipedia.org/wiki/Jack_Grubman
Keller’s comment that “The study authors comprised virtually all of the academic researchers studying the treatment of child depression in North America at the time” is an unwitting indictment of North American child psychiatry. The complicity was and remains pervasive and systemic.
Jeffrey Lieberman talking to Buzzfeed? APA’s public relations people are working overtime.
HA! Buzzfeed was denied credentials to cover the Pope’s visit!!!!! But Lieberman is A-OK with them! Too funny.
The internet is a like a modern day oracle, it’s also documents the historical record of mankind’s ideas and human thoughts and interaction, progress etc in real time , blogging facilitates this dynamic change in discourse as information evolves. Lieberman, Keller, GSK, Seroxat/Paxil and the rest, have been documented- how their ‘contributions’ to the understanding, evolution and progress in mental health discourse will be perceived in decades to come should prove very interesting indeed. They’re just too deluded and egotistical to realize the big picture now though. Drip Drip Drip… the info accumulates and self evidence materializes over time and no amount of sham hokus pokus tomfoolery can influence the power of the internet to see through bullshit. Keller and Lierberman are behaving with pre-internet brains, and they’re stuck in the late 20th century. Sorry guys but the world has moved on, people aren’t so easily fooled by your ilk anymore (putting on a white coat for a photo-shoot doesn’t wash either).
Dr. Nardo,
I’ve been following your blog for a few months now, and I suppose this is as good a time as any to express my gratitude for what you’ve been doing. I was heavily medicated as a kid—not Paxil, but Concerta, Zoloft, Risperdal, Zyprexa… I don’t remember them all, but they were good for nothing but side-effects. In hindsight, scientific misconduct and pharma influence explains my (mis)treatment better than any sound medical justification could. Perhaps if there’d been more voices like yours in the late 90’s and early 00’s I might not have lost a good bit of my adolescence to the medication, but it’s my hope the reanalysis leads to some healthy criticism and gives a few more kids the normal childhoods they deserve.
So—despite whatever criticism you and your colleagues may receive from the “experts”—from someone personally affected by this kind of corruption, you have my thanks.