by Jennifer E Miller, David Korn, and Joseph S RossBMJ Open 2015;5:e009758.
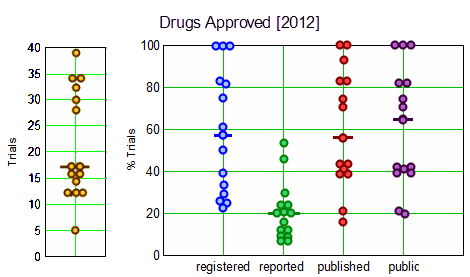
Objective: To evaluate clinical trial registration, reporting and publication rates for new drugs by: [1] legal requirements and [2] the ethical standard that all human subjects research should be publicly accessible to contribute to generalisable knowledge.Design: Cross-sectional analysis of all clinical trials submitted to the Food and Drug Administration [FDA] for drugs approved in 2012, sponsored by large biopharmaceutical companies.Data sources: Information from Drugs@FDA, ClinicalTrials.gov, MEDLINE-indexed journals and drug company communications.Main outcome measures: Clinical trial registration and results reporting in ClinicalTrials.gov, publication in the medical literature, and compliance with the 2007 FDA Amendments Acts [FDAAA], analysed on the drug level.Results: The FDA approved 15 drugs sponsored by 10 large companies in 2012. We identified 318 relevant trials involving 99,599 research participants. Per drug, a median of 57% [IQR 32–83%] of trials were registered, 20% [IQR 12–28%] reported results in ClinicalTrials.gov, 56% [IQR 41–83%] were published, and 65% [IQR 41–83%] were either published or reported results. Almost half of all reviewed drugs had at least one undisclosed phase II or III trial. Per drug, a median of 17% [IQR 8–20%] of trials supporting FDA approvals were subject to FDAAA mandated public disclosure; of these, a median of 67% [IQR 0–100%] were FDAAA-compliant. 68% of research participants [67,629 of 99,599] participated in FDAAA-subject trials, with 51% [33,405 of 67,629] enrolled in non-compliant trials. Transparency varied widely among companies.Conclusions: Trial disclosures for new drugs remain below legal and ethics standards, with wide variation in practices among drugs and their sponsors. Best practices are emerging. 2 of our 10 reviewed companies disclosed all trials and complied with legal disclosure requirements for their 2012 approved drugs. Ranking new drugs on transparency criteria may improve compliance with legal [FDAAA] and ethics standards and the quality of medical knowledge.
Transparency by legal standardsThere are at least three reasons why compliance with current disclosure laws might be suboptimal. First, legal requirements are perceived to be unclear or ambiguous, as a spectrum of interpretations of FDAAA has emerged…
Second, mergers, acquisitions, collaborations and licensing agreements may complicate compliance. Two companies in our sample acquired or licensed drugs initially developed by smaller companies, and another used a partner company for some trials, raising questions about whose responsibility it was to ensure trials complied with FDAAA.
Finally, compliance may be affected by a perceived lack of enforcement. FDAAA empowers the FDA to impose a $10,000 a day penalty for non-compliance. To date, this penalty has never been imposed.
Motivating transparencyGiven the wide variation in compliance with both legal and ethical standards across drugs and companies, we propose continuing our clinical trials transparency monitoring, evaluations and scoring of new drugs approved by the FDA, along with their sponsors. These ongoing rankings— developed initially with support from Harvard University, Duke University, Susan G. Komen Foundation and the Raskob Foundation [for a full list of sponsors, see the Acknowledgements section] — will be conducted annually under the auspicious of Bioethics International, with grant support from the Laura and John Arnold Foundation.
This system will help identify best practices, incent better behaviours and standardise the industry’s practices and thereby contribute importantly to an enrichment of medical knowledge. Moreover, the scorecard and rankings have the potential to benefit consumers of clinical trial information by helping to assure them of the integrity and completeness of their data. Not least, full transparency of clinical trials would also strengthen the protection of human research participants by avoiding their unknowing recruitment into already failed experiments.
Sorry, the comment form is closed at this time.