by Lawrence Scahill, M.S.N., Ph.D., James T. McCracken, M.D., Bryan H. King, M.D., Carol Rockhill, M.D., Bhavik Shah, M.D., Laura Politte, M.D., Roy Sanders, M.D., Mendy Minjarez, Ph.D., Jennifer Cowen, Ph.D., Jennifer Mullett, R.N., Chris Page, B.S., Denise Ward, M.A., Yanhong Deng, M.P.H., Sandra Loo, Ph.D., James Dziura, Ph.D., Christopher J. McDougle, M.D., and Research Units on Pediatric Psychopharmacology Autism NetworkAmerican Journal of Psychiatry. 2015 172[12]:1197-1206.
Objective: Hyperactivity, impulsiveness, and distractibility are common problems in children with autism spectrum disorder [ASD]. Extended-release guanfacine is approved for children with attention deficit hyperactivity disorder but not well studied in ASD.Method: In a multisite, randomized clinical trial, extended-release guanfacine was compared with placebo in children with ASD accompanied by hyperactivity, impulsiveness, and distractibility.Results: Sixty-two subjects [boys, N=53; girls, N=9; mean age=8.5 years SD=2.25] were randomly assigned to guanfacine [N=30] or placebo [N=32] for 8 weeks. The guanfacine group showed a 43.6% decline in scores on the Aberrant Behavior Checklist-hyperactivity subscale [least squares mean from 34.2 to 19.3] compared with a 13.2% decrease in the placebo group [least squares mean from 34.2 to 29.7; effect size=1.67]. The rate of positive response [much improved or very much improved on the Clinical Global Impression-Improvement scale] was 50% [15 of 30] for guanfacine compared with 9.4% [3 of 32] for placebo. A brief cognitive battery tapping working memory and motor planning showed no group differences before or after 8 weeks of treatment. The modal dose of guanfacine at week 8 was 3 mg/day [range: 1–4 mg/day], and the modal dose was 3 mg/day [range: 2–4 mg/day] for placebo. Four guanfacine-treated subjects [13.3%] and four placebo subjects [12.5%] exited the study before week 8. The most common adverse events included drowsiness, fatigue, and decreased appetite. There were no significant changes on ECG in either group. For subjects in the guanfacine group, blood pressure declined in the first 4 weeks, with return nearly to baseline by endpoint [week 8]. Pulse rate showed a similar pattern but remained lower than baseline at endpoint.Conclusions: Extended-release guanfacine appears to be safe and effective for reducing hyperactivity, impulsiveness, and distractibility in children with ASD.
The triad of hyperactivity, impulsiveness, and distractability is common in children with Autism [precise incidence is unclear, as under the DSM-IV, diagnosing ADHD in ASD was discouraged]. In ASD, the symptoms respond to ADHD treatment with stimulants, though side effects were greater than in ADHD. This group had done a small trial of Tenex® [Guanfacine] in ASD unresponsive to stimulants that was encouraging and decided to try Intuniv® in this trial. In the original clinicaltrial.gov version, there was an arm with Intuniv® plus Ritalin, but it disappeared somewhere along the way. The answer to my question, Why was this clinical trial funded with NIMH/NIH grant money rather than by Shire, the patent holder? was nowhere answered that I could find.
Supported by NIMH grants to Dr. Scahill (R01MH083707), Dr. McDougle (RO1MH83739), Dr. McCracken (RO1MH083747), and Dr. King (R01MH86927); by a Yale Clinical and Transitional Science Award (UL1 RR024139) from the NIH National Center for Research Resources; and by Atlanta Clinical and Translational Science Institute, Emory University, which is supported by the NIH National Center for Advancing Translational Sciences under award UL1TR000454. Shire Pharmaceuticals provided active extended-release guanfacine and placebo.
Description [R01MH083707]: This is a multi-site collaborative R01 application from The Research Units on Pediatric Psychopharmacology [RUPP] Autism Network (Indiana University, Seattle Children’s Research Institute, UCLA, and Yale University). Autism is a major public health concern throughout the world. The cost of the disability is estimated to be more than $30 billion annually in the U.S. alone. Recent data indicate that as many as 50% of children with pervasive developmental disorders (PDDs) have moderate to severe problems of hyperactivity and impulsiveness. The impact of these symptoms may be profound and make the child less able to make use of educational and behavioral interventions. Consensus is lacking on how to treat children with PDD accompanied by hyperactivity. Compared to typically developing children with ADHD, children with PDD often show less benefit and greater side effect burden. Guanfacine is commonly used in this population, but poorly studied. Our pilot data indicate that guanfacine is a promising treatment for hyperactivity in children with PDD with a good tolerability profile. In addition, we have identified biomarkers (genetic and neurochemical) that may be associated with positive effects. For these reasons, we chose guanfacine for the proposed rigorous and possibly definitive study in this population. The study involves an 8-week randomized, double-blind, placebo- controlled trial of guanfacine for the 170 children (ages 5-13 years) with PDD accompanied by hyperacti9vity and impulsiveness. Subjects who show a positive response will be invited to enter an 8-week Extension phase (treatment mask will not be broken). The treatment blind will be broken for children who do not achieve a positive response in the Double-blind phase. Children who show no change or deterioration on placebo will be treated with guanfacine in an 8-week Open-label phase. Children who show a partial response to guanfacine will be randomly assigned to a 4-week add on trial of methylphenidate or placebo to evaluate the potential benefits of combined treatment. We expect that 50 subjects will enter this pilot trial. The role of gene variants and urinary adrenergic/noradrenergic measures as biomarkers in moderating response to guanfacine on primary efficacy measures and adverse effects will be explored.
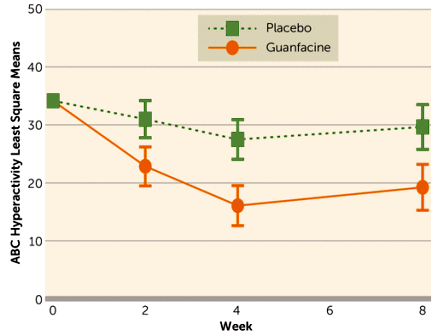
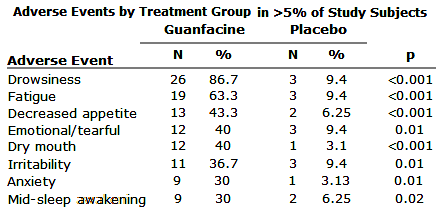
truncated to show only significant AEs
-
the PI has a significant COI [see below];
-
Intuniv® is in-patent and projected to be there for a while;
-
it could’ve been done with a generic [generic Guanfacine];
-
it reads like something a pharmaceutical rep might hand me. In fact, that pharmaceutical rep might say, "This is an NIMH study," implying that made it more legitimate, and would emphasize that it was done with the Extended Release [AKA in-patent] Intuniv®.
Different Topic:
Who is the most prominent, most politically powerful, voice acting as a counterpoint to this: “our enterprises in basic and clinical neuroscience, which is where the future of psychiatry resides.”
https://www.ucsf.edu/news/2015/06/130046/fresh-vision-fostering-mental-health-inspires-50-million-gift-ucsf
Especially the implication that this is where the near future (the eternal 5-10 year horizon) of our field resides.
Who has a platform to compete with the enormous amount of money supporting this message:”“There have been artificial separations between medicine, pediatrics, neurology and psychiatry for far too long. In addition to leading to poorer outcomes and higher costs, these separations have helped foster the stigma surrounding mental illness,” said State, whose research focus is on the genetics of autism, Tourette syndrome and related disorders. “With this landmark gift, we are going to break down these barriers, improve the care of our patients and their families, and help erase that stigma.””
I am not optimistic about the answers.
As a related aside, I would love to see a post that fleshed out your afterthought that:” I’d prefer teaching all health care workers basic interviewing skills as a more productive intervention…”
If integration is happening, and might even be a good thing in theory, how to assure that people like State & Lloyd Sederer are not the only ones steering that juggernaut?
http://www.newyorker.com/magazine/2011/03/21/the-poverty-clinic
Insel is the tip of the “Chasing the Rainbow” rainmakers.
‘“As we make progress in this area, the stigma and marginalization that are just part and parcel of having a psychiatric illness will start to fade, I guarantee you,” State says. “It is going to have an enormous impact.” ‘
https://www.ucsf.edu/news/2015/11/263881/illuminating-depressions-circuitry
It’s NOT that the research is not extremely important. It is. But it’s that the translational rhetoric, the looseness of the “depression” definitions, are swallowing up so much else.
How many of the hardcore translational clinical neuroscientists in the present are great clinicians?
What does “great clinician” mean to those in training today?
we need forums for discussing this not dominated by those who don’t see value in psychiatrists (which spans the “anti-psychiatrists” AND those who think that clinical neuroscience makes ‘psychiatry’ irrelevant).
Perhaps the MDD category, and the pre-eminence of rainmakers in psychiatry, are the original sins.
I don’t want to defend drug companies, the FDA or our whole system. Indeed, I’m one of those poor people who’s life was effectively ruined by an SSRI (which is how I find your blog: you wrote about SSRI protracted withdrawal sympathetically awhile back and I was impressed at your open-mindedness. Assuming it doesn’t happen to only a tiny minority of people (and I’d guess it occurs in a sizable minority but many don’t realize what is happening and just reinstate the drugs) this is going to one day come back and bite psychiatry in the ass, big time. ‘Torture’ doesn’t begin to describe the horror of that state… but forgive the incredibly long digression, I get few opportunities to vent my frustration about this. I wanted to correct you on one matter: I don’t know whether intuniv is still in patent or not but I can tell you first hand that generic formulations of it are available as of the last month or two. That might make it more appropriate for the NIH to be funding studies of it. I just started a trial of it myself for “primarily-inattentive adhd” or “sluggish cognitive tempo” or whatever psychiatry chooses to term it next. I’m not particularly optimistic since it has a reputation for only helping hyperactivity but I’m desperate as the stimulants which helped me function at a high level for years have slowly stopped working altogether- perhaps hence the ramble. Way more attention needs to be paid to the long term effects of medications.