I think I’ll borrow a literary device from a couple of favorites: T.S. Eliot in East Coker and John Fowles in The French Lieutenant’s Woman. It’s where the author jumps out of his story and speaks in the first person with the reader about his thoughts while he’s writing, then returns to the thread of the narrative…
When I first became belatedly aware of all the corrupted science and the industrial interference in our psychiatric literature, I was outraged and spent my time, like a lot of us, expressing my dissatisfaction, decrying and lamenting the sorry state of affairs. As I learned more, I figured out that a key feature of the problem was the lack of data transparency, and joined in that effort. I’ve been pleased that there has been some incremental movement in that regard from the collective efforts. But there’s something else. While I was pleased to be a part of finally getting the truth published about Paxil Study 329, it was 14 years after the fact when Paxil has been long off-patent [and hopefully mostly forgotten].
 I decided that what we need is part of a long-standing Preventive Medicine principle – Early Detection and Prompt Intervention. We need to jump on the Clinical Trials that are jury-rigged early, and stay on them from the very start before too much damage has been done. So on the first of every month, I scan through the journals and look for Clinical Trials that need vetting and get started. That’s how I got on this topic of Augmentation with Atypical Antidepressants in
I decided that what we need is part of a long-standing Preventive Medicine principle – Early Detection and Prompt Intervention. We need to jump on the Clinical Trials that are jury-rigged early, and stay on them from the very start before too much damage has been done. So on the first of every month, I scan through the journals and look for Clinical Trials that need vetting and get started. That’s how I got on this topic of Augmentation with Atypical Antidepressants in  Treatment Resistant Depression. And I’m following it as far as it will take me. I have no illusion that one old man can change much, but I’m hoping that some younger folks will catch the bug and take up the mantle. We’ll get nowhere if we don’t jump on these things quickly.
Treatment Resistant Depression. And I’m following it as far as it will take me. I have no illusion that one old man can change much, but I’m hoping that some younger folks will catch the bug and take up the mantle. We’ll get nowhere if we don’t jump on these things quickly.
So much for my little interlude coming clean about my agenda. It explains why I’ve taken to sticking in my amateur statistical pieces and why I plan some future looking-up-tips-and-tricks – passing on the tools of vetting I’ve picked up along the way. In fact, I’m about to pass one on right now – the wonders of a sometimes favorite website – Drugs@FDA.com…
When I found out that Brexpiprazole had been approved for the both Schizophrenia and Antidepressant Augmentation, I went straight to the FDA site [Drugs@FDA.com], but it wasn’t there. Sometimes, they lag and I wasn’t surprised. After I’d been through the papers, I’d worked up a good case of outrage, but it occurred to me that on my FDA trip, I’d only looked for brexpiprazole. Usually it’s under both names. But on a lark, I went back and looked under Rexulti®, and there it was! On that site, you look for the Approval History, Letters, Reviews, and Related Documents , then the Review, then the Medical Review(s) [all 238 pages!]. But don’t be discouraged, there’s usually a good table of contents for easy exploring. And there it was on page 95:
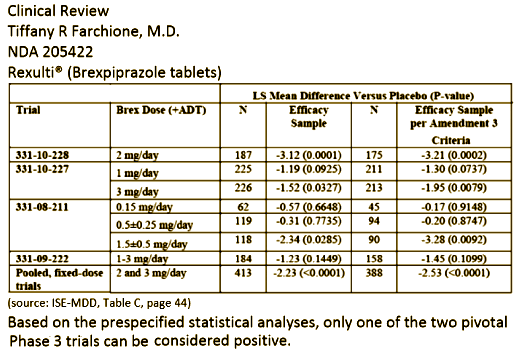
7.4. Adjunctive Treatment of MDD
7.4.1. The Sponsor conducted two adequate and well – controlled trials to asse ss the efficacy of brexpiprazole for the adjunctive treatment of MDD. Based on the prespecified statistical analysis plan, only one of these trials (Study 331-10-228) was positive. The Sponsor acknowledges that Study 331-10-227 was not positive based on the pre-specified plan, but provides a number of arguments to support the concept that brexpiprazole should nonetheless be approved for this indication.
Approval of the adjunctive MDD indication would have to rest on one of two possible scenarios: 1. Study 331-10-227 can be considered a positive trial based on the retrospective application of Amendment 3 criteria and use of the per protocol population instead of the intent – to – treat population for the primary analysis. 2. Study 331-10-228 can be considered “strongly positive” and, thus, approval can be based on this single study. Study 331-10-227 would then be viewed as “supportive evidence” for this approval.
In the first scenario, it is true that efficacy of brexpiprazole 3 mg/day was demonstrated in Stud y 331 – 10 – 227 using the per protocol population. When Amendment 3 was implemented, the data were still blinded. At that time, the Sponsor assured the Division that the primary analysis would still be based on the ITT population and did not request any modification to the statistical analysis plan. Only once the data were unblinded and the “near miss” was discovered did the Sponsor ask us to consider these alternative analyses. There is no question that Amendment 3 resulted in randomization of more appropriate subjects; it is the retrospective application of the amendment criteria to unblinded data that is problematic. This may be a case in which hindsight is 20/20 — had the Sponsor asked to modify the statistical analysis plan to use the per protocol population for the primary efficacy analysis, we may have granted that request. There is precedent for such decisions as long as the blind has not been broken. But, in this case, no such request was made. Changing the statistical analysis plan after the blind has be en broken is not appropriate ; thus, we cannot simply accept this as a positive trial.
On the other hand , the results of Study 331-10-227 can be viewed as supportive evidence, allowing for approval based on the second scenario. As noted above, there is no question that Amendment 3 resulted in randomization of more appropriate subjects, and no question that this would have been a positive study if the Sponsor had used the Amendment 3 criteria from the start of the study. Indeed, if one restricts the analysis of 331-10-227 to only those subjects randomized after Amendment 3 (taking the question of blinded vs. unblinded selection of subjects for analyses off the table), the 3 mg/day brexpiprazole+ADT would be statistically superior to placebo+ADT (p=0.003 3 ).
In addition to these considerations , the Sponsor picked a particularly stringent statistical method for dealing with multiple comparisons — a method which we advised against. If the Sponsor had chosen a less stringent method (for instance, testing 3 mg first then 1 mg), the study would have been positive. The Sponsor also employed the Hochberg procedure in Study 331-08-211. Similar to Study 331-10-227, one dose group (1.5 + 0.5 mg/day) in 331-08-211 yielded a p<0.05 (p=0.0285) on the primary endpoint. But, al so similar to 331-10-227, the pre – specified multiplicity adjustment required a lower threshold. Of note, a retrospective application of Amendment 3 criteria to the study population in 331-08-211 would also make the treatment effect in this dose group statistically superior to placebo (p=0.0092).
One can also reasonably consider Study 331-10-228 a “strongly positive” study — in a population of individuals with a history of multiple failed antidepressant trials and prospectively demonstrated suboptimal respons e to an additional antidepressant, the addition of 2 mg/day of brexpiprazole yielded an average additional symptom improvement of just over 8 points on the MADRS. This was 3 points beyond the improvement in placebo — a difference that was highly statistically significant at p=0.0001. These results are both clinically and statistically meaningful.
Thus, with one strongly positive trial and supportive evidence from two additional trials, there is adequate evidence of efficacy to approve this product for the adjunctive treatment of MDD.
Thank you for the link to Drugs@FDA.com
(https://www.accessdata.fda.gov/scripts/cder/drugsatfda/)
I sometimes happen upon it but I don’t always find it when I look for it.
An improvement of 3 MADRS points beyond placebo seems like a relatively small magnitude considering the overall score range of 0 to 60.