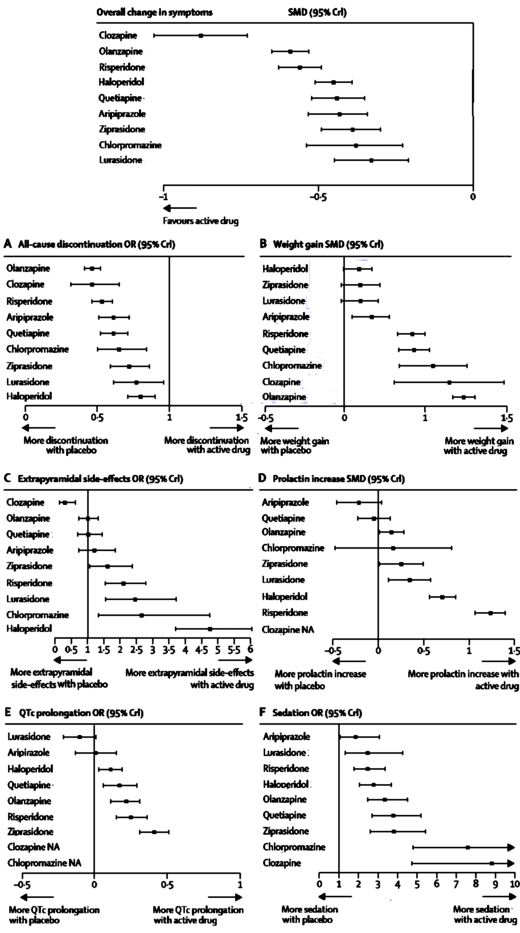
So there’s an article that I’ve looked up over and over. I referred to it a lot looking into Brexpiprazole. It compares the strength of effect of various parameters for the Atypical Antipsychotics. I thought it was a shame it wasn’t open access, so I went after its forest plots with my graphics program and removed all but the bare bones [including the drugs not available in the US]. After I got through, I went to post it and found that there was a pdf available on-line after all. Oh well. My summary graphic is below anyway…
by Stefan Leucht, Andrea Cipriani, Loukia Spineli, Dimitris Mavridis, Deniz Örey, Franziska Richter, Myrto Samara, Corrado Barbui, Rolf R Engel, John R Geddes, Werner Kissling, Marko Paul Stapf, Bettina Lässig, Georgia Salanti, and John M DavisThe Lancet. 2013 382:951-962.
Background The question of which antipsychotic drug should be preferred for the treatment of schizophrenia is controversial, and conventional pairwise meta-analyses cannot provide a hierarchy based on the randomised evidence. We aimed to integrate the available evidence to create hierarchies of the comparative efficacy, risk of all-cause discontinuation, and major side-effects of antipsychotic drugs.Methods We did a Bayesian-framework, multiple-treatments meta-analysis [which uses both direct and indirect comparisons] of randomised controlled trials to compare 15 antipsychotic drugs and placebo in the acute treatment of schizophrenia. We searched the Cochrane Schizophrenia Group’s specialised register, Medline, Embase, the Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov for reports published up to Sept 1, 2012. Search results were supplemented by reports from the US Food and Drug Administration website and by data requested from pharmaceutical companies. Blinded, randomised controlled trials of patients with schizophrenia or related disorders were eligible. We excluded trials done in patients with predominant negative symptoms, concomitant medical illness, or treatment resistance, and those done in stable patients. Data for seven outcomes were independently extracted by two reviewers. The primary outcome was efficacy, as measured by mean overall change in symptoms. We also examined all-cause discontinuation, weight gain, extrapyramidal side-effects, prolactin increase, QTc prolongation, and sedation.Findings We identifi ed 212 suitable trials, with data for 43 049 participants. All drugs were significantly more effective than placebo. The standardised mean differences with 95% credible intervals were: clozapine 0·88, 0·73–1·03; amisulpride 0·66, 0·53–0·78; olanzapine 0·59, 0·53–0·65; risperidone 0·56, 0·50–0·63; paliperidone 0·50, 0·39–0·60; zotepine 0·49, 0·31–0·66; haloperidol 0·45, 0·39–0·51; quetiapine 0·44, 0·35–0·52; aripiprazole 0·43, 0·34–0·52; sertindole 0·39, 0·26–0·52; ziprasidone 0·39, 0·30–0·49; chlorpromazine 0·38, 0·23–0·54; asenapine 0·38, 0·25–0·51; lurasidone 0·33, 0·21–0·45; and iloperidone 0·33, 0·22–0·43. Odds ratios compared with placebo for all-cause discontinuation ranged from 0·43 for the best drug [amisulpride] to 0·80 for the worst drug [haloperidol]; for extrapyramidal side-eff ects 0·30 [clozapine] to 4·76 [haloperidol]; and for sedation 1·42 [amisulpride] to 8·82 [clozapine]. Standardised mean differences compared with placebo for weight gain varied from –0·09 for the best drug [haloperidol] to –0·74 for the worst drug [olanzapine], for prolactin increase 0·22 [aripiprazole] to –1·30 [paliperidone], and for QTc prolongation 0·10 [lurasidone] to –0·90 [sertindole]. Efficacy outcomes did not change substantially after removal of placebo or haloperidol groups, or when dose, percentage of withdrawals, extent of blinding, pharmaceutical industry sponsorship, study duration, chronicity, and year of publication were accounted for in meta-regressions and sensitivity analyses.Interpretation Antipsychotics differed substantially in side-effects, and small but robust diff erences were seen in effi cacy. Our findings challenge the straightforward classification of antipsychotics into first-generation and second- generation groupings. Rather, hierarchies in the different domains should help clinicians to adapt the choice of antipsychotic drug to the needs of individual patients. These findings should be considered by mental health policy makers and in the revision of clinical practice guidelines.Funding None
1bom, As an author of a Pubmed indexed publication you can append comments to the Pubmed listing of these papers. Have you considered developing a penultimate document about one or more of these papers and linking to it (or directly including the info if links aren’t allowed) in such a comment? That way anyone coming across the abstract gets to see it.
I mean, those 2 Thase papers are just crying out for PubMed Commons comments, no? None appear with them so far.
A final thought for the night:
http://motherboard.vice.com/read/how-ketamine-infusions-saved-my-life
http://www.ketamineadvocacynetwork.org/2013/07/09/welcome-to-the-ketamine-advocacy-network/
http://www.ketaminenetwork.org/provider-directory/