I suppose a "pardon my French" apology is in order for my outburst in the last post on seeing that Rexulti® television ad yesterday. I’m not big on profanity and I apologize, but sometimes bold, underlined, italicized , or multiple repetitive posts just aren’t loud enough. As I’ve acquainted myself with what I think of as these tainted studies, most of the really egregious stuff has been in the past, a Paxil Study 329 or a Seroquel Study 15, things that happened a long time ago. I think I had the fantasy that if one could catch them early, they could be headed off. But in this case, that’s apparently just a fantasy. Here’s the ad that evoked that response:
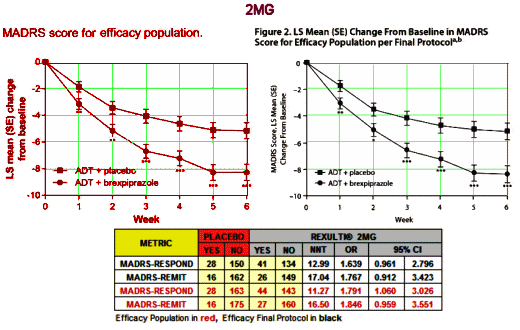
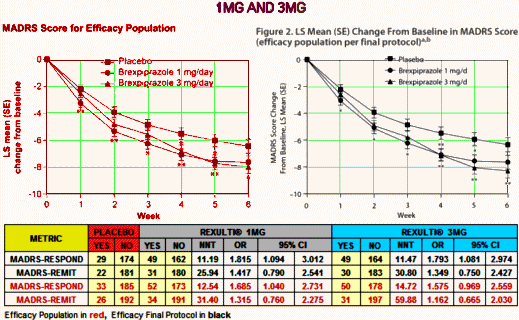
Major Depressive Disorder isn’t an entity, a noun. In practice it’s an adjective describing a feeling. Its variant, Treatment Resistant Depression, is a tautology, even less a noun, more an adverb modifying the verb to treat. Yet the FDA has elevated it to a category, an entity for drug approval. And they went along with approving Rexulti®, a clone of the antipsychotic Abilify [just off patent], for Treatment Resistant Depression in spite of having only one study that achieved their minimal standards [statistical significance]. The second required study decidedly did not, unless you smeared your lens with vasoline and fell for some outrageous sleight of hand in plain view. If you look up the cost of Rexulti® on the Internet [which lists costs with a discount coupon], it sells for about $30/pill. And Abilify, the drug it replaces in-patent, is generic but still costs ~$10/pill.
If you are a prescriber or a patient who is tempted by that ad, at least read the material in the last post and look at the NNTs before you leap. Also, for the mathematically inclined, take a look at john henry’s hammer: continuous variables III…:
Abilify generic 10 mg is about $5 per pill ordered from Canada. You can split them in half also.