" Today the U.S. Senate voted in support of the confirmation of Dr. Robert Califf, M.D. to be Commissioner of U.S. Food and Drug Administration. Dr. Califf has demonstrated a long and deep commitment to advancing the public health throughout his distinguished career as a physician, researcher, and leader in the fields of science and medicine. He understands well the critical role that the FDA plays in responding to the changes in our society while protecting and promoting the health of the public, across the many areas we regulate – and I am confident that our public health and scientific contributions will further grow under his exceptional leadership."
Today the U.S. Senate voted in support of the confirmation of Dr. Robert Califf, M.D. to be Commissioner of U.S. Food and Drug Administration. Dr. Califf has demonstrated a long and deep commitment to advancing the public health throughout his distinguished career as a physician, researcher, and leader in the fields of science and medicine. He understands well the critical role that the FDA plays in responding to the changes in our society while protecting and promoting the health of the public, across the many areas we regulate – and I am confident that our public health and scientific contributions will further grow under his exceptional leadership."
 At some other point, we might not have paid a lot of attention or even noticed the appointment of a new FDA Commissioner. For that matter, I wonder if we would have been aware that the position of Director of the NIMH has just been vacated. But right now, both have been on the front burner. Besides their obvious centrality in the ‘hot topic’ of pharmaceuticals, a prime subject in our daily news, there’s something else that connects them – their relationship to the information age and its big data. Both are intrigued by and involved in the application of these new technologies to how we do medical research and specifically how they relate to the clinical trials we use to evaluate medications.
At some other point, we might not have paid a lot of attention or even noticed the appointment of a new FDA Commissioner. For that matter, I wonder if we would have been aware that the position of Director of the NIMH has just been vacated. But right now, both have been on the front burner. Besides their obvious centrality in the ‘hot topic’ of pharmaceuticals, a prime subject in our daily news, there’s something else that connects them – their relationship to the information age and its big data. Both are intrigued by and involved in the application of these new technologies to how we do medical research and specifically how they relate to the clinical trials we use to evaluate medications.
I called Insel a breakthrough freak, and I think I’m right about that. He was always in the future, aiming for the next big thing, constantly grumbling about the present. And that kind of thinking might sometimes be a real asset in a researcher. In his case, it didn’t seem balanced by the problems of the present – and so his blogs, speeches, and programs were always aiming for home runs instead of just trying to get on base. The other thing that bothered me was that he never practiced medicine – and it showed. His thinking often reminded me of my own when I was a medical student – uninformed by the clinical experience that builds a grounded medical intuition, something I thought he lacked.
Dr Califf currently holds the post of Deputy Commissioner for Medical Products and Tobacco, US Food and Drug Administration. Prior to holding this post, Dr Califf received grant funding from the Patient-Centered Outcomes Research Institute, the National Institutes of Health, the US Food and Drug Administration, Merck, Roche, Aterovax, Bayer, Janssen Pharmaceuticals, Eli Lilly & Company, and Schering-Plough; grants and personal fees from Novartis, Amylin, Scios, and Bristol-Myers Squibb/Bristol-Myers Squibb Foundation; and personal fees from WebMD, Kowa Research Institute, Nile, Parkview, Orexigen, Pozen, Servier International, Bayer Healthcare, Bayer Pharma AG, CV Sight, Daiichi Sankyo/Lilly, Gambro, Gilead, Heart.org–Bayer, Medscape, Pfizer, Regeneron, TMC, GlaxoSmithKline, Genentech, Heart.org–Daiichi Sankyo, and Amgen. Dr Califf also reported holding equity in Nitrox/N30 and Portola. A full listing of disclosure information for Dr Califf for this interval is available at https://www.dcri.org/about-us/ conflict-of-interest.
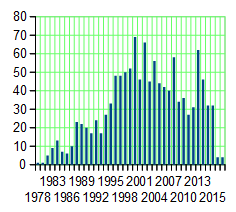 He’s certainly well represented in our medical literature. His name is on over 1100 articles indexed in PubMed, going back to 1978 when he was young [64-38=26]. The ordinate [y axis] on the chart represents his articles/year [articles/year!!!]. He was involved in starting the Duke Databank for Cardiovascular Disease in 1983 and was the founding director of the Duke Clinical Research Institute in 1996. He authored a particularly telling short editorial way back at the beginning of that graph [1981] that’s worth a read in that it seems to presage his subsequent career – The Doctor and the Computer [for reference: that’s the year the IBM PC was introduced]. The point being that he’s a computer/data guy who got on that bus from his earliest of days.
He’s certainly well represented in our medical literature. His name is on over 1100 articles indexed in PubMed, going back to 1978 when he was young [64-38=26]. The ordinate [y axis] on the chart represents his articles/year [articles/year!!!]. He was involved in starting the Duke Databank for Cardiovascular Disease in 1983 and was the founding director of the Duke Clinical Research Institute in 1996. He authored a particularly telling short editorial way back at the beginning of that graph [1981] that’s worth a read in that it seems to presage his subsequent career – The Doctor and the Computer [for reference: that’s the year the IBM PC was introduced]. The point being that he’s a computer/data guy who got on that bus from his earliest of days.He’s a Cardiologist, a specialty that’s unrecognizable from my time as a medical resident in the late 1960s. Along with the cardiovascular surgeons, they’ve revolutionized the treatment of heart disease. And he’s been a part of that to his credit. I personally think they’re a too quick to jump in with some of their implantable devices, and their recommendations about Statins are way out of line. I actually feel the same way about Xarelto®. As often as it’s touted as a breakthrough, I see it as only a drug of convenience, and if I develop Atrial Fibrillation like so many of my current peers, I think I’ll stick with Warfarin for myself. Sure enough, there are dietary and medication restrictions, and it needs to be monitored, but you can turn it off. You can’t turn Xarelto® off. One car wreck, and you’re in deep trouble, or a GI bleed, or just being old [right now, I have a 95 year old colleague in the hospital for dangerous spontaneous bleeding on one of Xarelto®‘s competitors]. Monitoring Warfarin just isn’t that hard. But in the main, the progress in cardiovascular medicine is impressive and then some.
But oddly, none of that is what really bothers me about his appointment. Right now there are two opposing critiques of the FDA. Perhaps the loudest is the cry for more drugs and the FDA is seen as a bottleneck in that process. PHARMA is pushing for a more streamlined process to make approval faster and easier. And the world has a voracious appetite for new drugs and is pulling for for the same thing – more, faster, easier. One of the ways people are thinking about doing that is to utilize the wealth of Electronic Medical Record information and the techniques of Big Data to evaluate pharmaceuticals. Dr. Califf has been in the center of that move, recently the editor of an issue of Clinical Trials devoted to pragmatic clinical trials, a version of that genre. Who hasn’t had the fantasy of doing clinical trials as part of ongoing medical care using these newer resources and techniques? I certainly have, particularly in monitoring long term efficacy and safety.
But, what about the CNS drugs? What about all the RCTs done on the antidepressants and antipsychotics that have such shaky science? What about approving Brexpiprazole for treatment resistant depression, or for that matter, any drug for treatment resistant depression? What about that misleading ad I can’t stop talking about? What about HHS and SAMHSA pushing for Behavioral Health moving to primary care with Collaborative Care as a backup, psychiatrists suggesting drug regimens for patients they haven’t even seen. What about waiting room screening – sure to escalate the inappropriate overmedication of patients? The FDA has certainly had its part in colluding with the pharmaceutical industry whether on purpose or inadvertently in all of these things. It took them seventeen years to add a black box warning that was needed after only a few.
The charge of the FDA is to insure the safety and effectiveness of our pharmacopeia, and in my corner of the world [psychiatry], the net effect has been disappointing at times, and scandalous at others. Many of us have asked for something we should have had all along – Data Transparency – the right to look independently at the raw data from clinical trials and reach our own conclusions instead of the fiction of the ghost writers. Guido Rasi and others at the European Medicines Agency have worked at moving us in that direction, but the FDA has not except for occasional rhetorical bursts that invariably fade. And we know that in spite of all of our complaints about short term RCTs, we still need them. Population and "naturalistic" studies of harms and efficacy haven’t worked out for us so far. There’s just no way to say that the FDA has provided effective mechanisms that guarantee safety and efficacy with our medications. They’ve stuck with minimal statistical significance for Efficacy and short term Adverse Event analysis – failing to roll up their sleeves and address the obvious problems that are still with us. The legal record of PHARMA penalties and settlements is in part a monument to those failings.
Sorry, the comment form is closed at this time.