- Medicine/Medical. characteristic or diagnostic of a specific disease.
-
anything to indicate or point out, as a sign or token.
-
Medicine/Medical. a special symptom or the like that points out a suitable remedy or treatment or shows the presence of a disease.
-
an act of indicating.
-
the degree marked by an instrument.
I didn’t see detail men in practice, but my partners did. So occasionally, I would get caught. In one such captivity, the sales rep looked at me meaningfully and said with emphasis, "We’ve just found out that we have a new indication for whatever-drug-it-was in whatever-condition-it-was" [as if that had a very special, near mystical significance]. I had never heard it used that way [my ignorance a consequence of chronic avoidance of sales reps]. Later, I asked one of my partners, who explained that the FDA approved drugs for specific diseases or situations. I supposed that was a reasonable idea, a way of communicating what they looked at when they put the drug on the market. I later asked her, "Have they always done that?" She laughed at my by-then legendary naivity in such matters and said, "I don’t know, but what it really means is that they can advertise it for that indication" [truth comes in many forms, and that’s an example].
-
Medicine/Medical. a special symptom or the like the FDA certifies for a pharmaceutical company’s marketing department to legalize their advertising it as a use for their drug.
Panel debates vortioxetine for treating cognitive dysfunction in major depressionMEDPAGE TODAYby Kristina Fiore02/03/2016An FDA advisory committee voted 8-to-2 to give the antidepressant vortioxetine [Brintellix] a new indication for cognitive dysfunction in major depressive disorder [MDD]. The decision followed an unusual meeting format for the Psychopharmacologic Drugs Advisory Committee, with the first half of the day dedicated to discussing whether or not cognitive dysfunction in MDD is a suitable target for development, and if so, how exactly it should be studied…
Historically, the FDA’s division of psychiatry products within Center for Drug Evaluation and Research has taken the position that cognitive dysfunction in MDD was a pseudo-specific drug target, "meaning that this claim would be considered artificially narrow and related to the overall disorder of MDD," the agency wrote in review documents posted ahead of the meeting. But the organization changed its stance as more research has suggested that it can be considered a distinct problem that hasn’t been evaluated in drug trials.
Starting in 2011, Lundbeck, the original developer of vortioxetine, began submitting data on the drug’s efficacy in cognitive dysfunction to the FDA. In 2014, Takeda and Lundbeck met with FDA to further discuss the potential indication, and the agency ultimately decided on a public hearing to discuss both the possibility of such an indication and the efficacy of the drug on the same day…
In one of the approval trials, called ELDERLY, vortioxetine offered better results on the digit symbol substitution test [DSST] than duloxetine, prompting the companies to open two other trials focused on cognitive impairment in MDD: FOCUS and CONNECT, both of which enrolled 602 patients for an 8-week trial period. FOCUS looked at a composite of DSST plus Rey Auditory Verbal Learning Test [RAVLT] learning and memory for its primary endpoint, while CONNECT looked only at DSST — and both showed an effect, albeit a small one, according to FDA clinical reviewer Wen-Hung Chen, PhD…
Should the FDA decide that cognitive dysfunction in MDD is indeed a valid therapeutic target and that Takeda has demonstrated that vortioxetine improves the condition, the two groups will work together to define the exact terms of the labeling. The agency is not obliged to follow recommendations from its advisory committees, but it usually does.
[see also Medscape FDA Panel Backs Vortioxetine for Cognitive Dysfunction in MDD]
by McIntyre RS, Lophaven S, and Olsen CK.International Journal of Neuropsychopharmacology. 2014 17[10]:1557-1567.Clinical Trial NCT01422213
The efficacy of vortioxetine 10 and 20 mg/d vs. placebo on cognitive function and depression in adults with recurrent moderate-to-severe major depressive disorder [MDD] was evaluated. Patients [18-65 yr, N = 602] were randomized [1:1:1] to vortioxetine 10 or 20 mg/d or placebo for 8 wk in a double-blind multi-national study. Cognitive function was assessed with objective neuropsychological tests of executive function, processing speed, attention and learning and memory, and a subjective cognitive measure. The primary outcome measure was change from baseline to week 8 in a composite z-score comprising the Digit Symbol Substitution Test [DSST] and Rey Auditory Verbal Learning Test [RAVLT] scores. Depressive symptoms were assessed using the Montgomery-Åsberg Depression Rating Scale [MADRS]. In the pre-defined primary efficacy analysis, both doses of vortioxetine were significantly better than placebo, with mean treatment differences vs. placebo of 0.36 [vortioxetine 10 mg, p < 0.0001] and 0.33 [vortioxetine 20 mg, p < 0.0001] on the composite cognition score. Significant improvement vs. placebo was observed for vortioxetine on most of the secondary objectives and subjective patient-reported cognitive measures. The differences to placebo in the MADRS total score at week 8 were -4.7 [10 mg: p < 0.0001] and -6.7 [20 mg: p < 0.0001]. Path and subgroup analyses indicate that the beneficial effect of vortioxetine on cognition is largely a direct treatment effect. No safety concern emerged with vortioxetine. Vortioxetine significantly improved objective and subjective measures of cognitive function in adults with recurrent MDD and these effects were largely independent of its effect on improving depressive symptoms.
by Atul R Mahableshwarkar, John Zajecka, William Jacobson, Yinzhong Chen and Richard SE KeefeNeuropsychopharmacology. 2015 40: 2025–2037.Clinical Trial NCT01564862
This multicenter, randomized, double-blind, placebo-controlled, active-referenced [duloxetine 60 mg], parallel-group study evaluated the short-term efficacy and safety of vortioxetine [10-20 mg] on cognitive function in adults [aged 18-65 years] diagnosed with major depressive disorder [MDD] who self-reported cognitive dysfunction. Efficacy was evaluated using ANCOVA for the change from baseline to week 8 in the digit symbol substitution test [DSST]-number of correct symbols as the prespecified primary end point. The patient-reported perceived deficits questionnaire [PDQ] and physician-assessed clinical global impression [CGI] were analyzed in a prespecified hierarchical testing sequence as key secondary end points. Additional predefined end points included the objective performance-based University of San Diego performance-based skills assessment [UPSA] [ANCOVA] to measure functionality, MADRS [MMRM] to assess efficacy in depression, and a prespecified multiple regression analysis [path analysis] to calculate direct vs indirect effects of vortioxetine on cognitive function. Safety and tolerability were assessed at all visits. Vortioxetine was statistically superior to placebo on the DSST [P < 0.05], PDQ [P < 0.01], CGI-I [P < 0.001], MADRS [P < 0.05], and UPSA [P < 0.001]. Path analysis indicated that vortioxetine’s cognitive benefit was primarily a direct treatment effect rather than due to alleviation of depressive symptoms. Duloxetine was not significantly different from placebo on the DSST or UPSA, but was superior to placebo on the PDQ, CGI-I, and MADRS. Common adverse events [incidence ? 5%] for vortioxetine were nausea, headache, and diarrhea. In this study of MDD adults who self-reported cognitive dysfunction, vortioxetine significantly improved cognitive function, depression, and functionality and was generally well tolerated.
Primary Analysis
Based on the ANCOVA analysis, the change from baseline [mean±SE] to week 8 in DSST performance score was 4.60±0.53 for vortioxetine, 4.06±0.51 for duloxetine, and 2.85±0.54 for placebo.The difference from placebo was significant for vortioxetine [Δ +1.75, 95% CI: 0.28, 3.21; P=0.019; ANCOVA, OC], with a standardized effect size of 0.254. The difference from placebo was not significant for the duloxetine group [Δ +1.21, 95% CI: −0.23, 2.65; P=0.099], with a standardized effect size of 0.176.
Depression Outcome
The study was validated because both vortioxetine and duloxetine demonstrated a statistically significant change from baseline in mood symptoms compared with placebo at the end of week 8, as measured by change in MADRS [vortioxetine, Δ − 2.3, 95% CI: − 4.3, − 0.4; P < 0.05; duloxetine, Δ − 3.3, 95% CI: − 5.2, − 1.4; P < 0.001; MMRM, FAS].
The end game is getting an indication (or even off-label buzz) for dementias and other cognitive disorders. This was pretty inevitable with Brintellix since it is said to work through five different neurotransmitter systems. So if the existing drugs (that don’t work well) work through acetylcholine, therefore…(never mind the data.)
I served on the FDA Advisory Committee during the 1980s. Based on that experience, I see troubling issues in the Keefe report.
1. Nominal statistical significance was seen for only 3 of 11 primary and secondary cognitive tests in the vortioxetine group. That makes it hard to claim a global effect on cognition in these depressed patients. The major inconsistencies among the items in the cognitive test battery are unexplained.
2. There were a few extra nominally significant findings among the Additional Endpoints but these would wash out with Bonferroni correction. That would include the MADRS depression efficacy analysis, by the way, which had a nominal efficacy of only p = 0.02. So, even the claim that vortioxetine had an antidepressant effect in these subjects is not really supported by the data.
3. Against that background, the claim from path analysis that the effect on DSST was independent of an antidepressant effect is meaningless.
4. The observed effect did not meet the projection of their power analysis for the primary end point of change in DSST cognitive performance. The actual group difference observed between vortioxetine and placebo was only 1.75 on the DSST – just 53% of the projected difference.
5. Most importantly, the clinical significance of the DSST results is nugatory. The median baseline DSST score was 44, and a baseline score above 70 was used to disqualify patients during screening. Against those benchmarks, the observed mean DSST improvement difference of just 1.75 points between vortioxetine and placebo is pitiful, just pitiful, no matter what the nominal statistical significance. And that was after 8 weeks!
Based on comments made by the authors in the Discussion, I can only agree with James O’Brien – the ultimate target for Takeda is probably dementing disorders as “new indications” for vortioxetine. One can only hope that the FDA turns them down on this trial balloon of cognitive impairment in major depression. The data are underwhelming.
Thanks to both of you for stepping up to the plate on this. This indication could really add to the overmedication problem, particularly in the elderly. In the MEDPAGE article, the reporter said, “Concerns raised about expansion of an antidepressant’s label to include cognitive dysfunction include a race among other antidepressants to win the same claim, as well as worry that switching patients off of their current antidepressants to try a new one could cause problems.” I presume that came from the hearing and they’re exactly right. There’s little question that the MDD diagnosis needs to be fractionated, but treatment resistance in… or cognitive dysfunction in… are hardly the right ways to go about that task. They’re tautologies, not diagnoses [or even subdiagnoses]…
Addendum: On scanning the Supplementary materials available on-line, I see in Appendix B that response rates and remission rates were specified as outcome variables. That information is completely missing from the published report, however. This confirms my impression that the analyses were cherry-picked to favor the company, Takeda.
Trails B takes usually no more than a minute and a half. Im my experience there are few false negatives but plenty of false positives in normals who are simply tired at that point in the exam.
to clarify: negative means intact, there are many reasons for a positive test…it’s a pretty crude measure of cognitive impairment and is effort dependent…
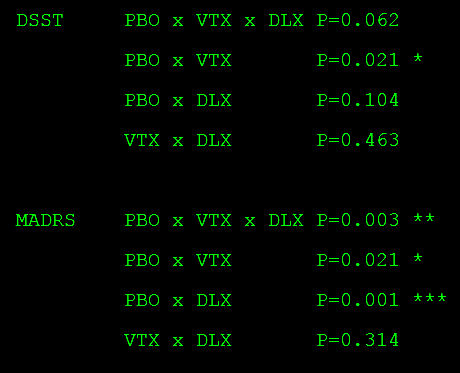
I can confirm your overall ANOVA result with p = 0.062, nonsignificant. In addition, the normal bivariate post hoc testing is not significant for any comparison of the change in DSST scores (below). Isn’t it nice to be able to scrutinize analyses with the reported data?
Tukey HSD Post-hoc Test…
PLA Group vs VTX Group: Diff=1.7500, 95%CI=-0.0262 to 3.5262, p=0.0545
PLA Group vs DLX Group: Diff=1.2100, 95%CI=-0.5381 to 2.9581, p=0.2354
VTX Group vs DLX Group: Diff=-0.5400, 95%CI=-2.2669 to 1.1869, p=0.7429