-
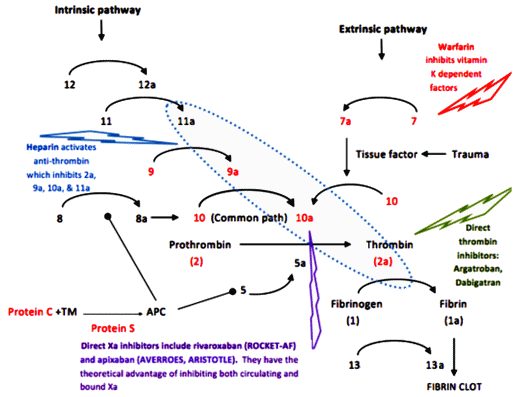
Heparin – a naturally occurring compound found in certain cells. As a drug, it has to be given by injection, acts immediately, is short acting [hours], and blocks the action of any number of clotting factors. It can be reversed [with Protamine Sulfate].
-
Warfarin [Coumadin] – Originally introduced as a Rat Poison, Warfarin interferes with the the metabolism [re-use] of Vitamin K which is necessary for the creation and action of a number of those factors [in red], specifically their binding of Calcium. It takes several days to work [produce a Vitamin K deficient state], is sensitive to dietary factors and many drugs, and has to be monitored with frequent [monthly] blood tests. It can be fairly quickly reversed with Vitamin K injections.
A few years back, I ran across something new. A friend [with limited means] developed Atrial Fibrillation and was prescribed a drug I’d never heard of before. He was fretting over the cost, and I looked into it. It was one of the new ones, Pradaxa®, and it would’ve been a big out-of-pocket expense for a retired "man of the cloth." He was told that there weren’t the restrictions and you didn’t have to get all of those blood tests. It was "better." So I hit the Internet and learned about Xarelto® and Pradaxa®. The restrictions, the hassle of the blood tests, the dosage adjustments weren’t necessary sure enough, but underscore avoid having a big car wreck! two or three times, because at the time I was looking, there was no easy antidote except time. In the figure, their action is more specific, way down at the bottom of the cascade – and there was no off switch then
We had endlessly teased my friend and his wife, calling them Gypsies, because they were always on the road visiting many friends. But his doctor got him a discount somehow, so he opted for the new drug and had no problems, later dying from something unrelated. But in my searching around, I decided that these were "drugs of convenience" rather than "better." I have to admit that my negativity probably has to do with having spent some long nights when I was younger with several wreck victims on Coumadin who I doubt would’ve survived without that off switch.
-
New York Timesby Katie ThomasMarch 1, 2016
-
by Manesh R. Patel, Kenneth W. Mahaffey, Jyotsna Garg, Guohua Pan, Daniel E. Singer, Werner Hacke, Günter Breithardt, Jonathan L. Halperin, Graeme J. Hankey, Jonathan P. Piccini, Richard C. Becker, Christopher C. Nessel, John F. Paolini, Scott D. Berkowitz Keith A.A. Fox, Robert M. Califf, M.D., and the ROCKET AF Steering Committee, for the ROCKET AF InvestigatorsNew England Journal of Medicine 2011 365:883-891.
-
Alere INRatio2 PT/INR Professional Test Strips: recall — higher INR when performed by central laboratory Food and Drug Administration. December 5, 2014
-
BMJ 2015 351:h6431
-
by Manesh R. Patel, Anne S. Hellkamp, Keith A. A. Fox, for the ROCKET AF Executive Committee and InvestigatorsNew England Journal of Medicine. 2016 374:785-788.
-
New York Timesby Katie ThomasFebruary 22, 2016
New York TimesBy KATIE THOMASMARCH 1, 2016It is a startling accusation, buried in a footnote in a legal briefing filed recently in federal court: Did two major pharmaceutical companies, in an effort to protect their blockbuster drug, mislead editors at one of the world’s most prestigious medical journals? Lawyers for patients suing Johnson & Johnson and Bayer over the safety of the anticlotting drug Xarelto say the answer is yes, claiming that a letter published in The New England Journal of Medicine and written primarily by researchers at Duke University left out critical laboratory data. They claim the companies were complicit by staying silent, helping deceive the editors while the companies were in the midst of providing the very same data to regulators in the United States and Europe…
Duke and Johnson & Johnson contend that they worked independently of each other. Bayer declined to comment. And top editors at The New England Journal of Medicine said they did not know that separate laboratory data existed until a reporter contacted them last week, but they dismissed its relevance and said they stood by the article’s analysis. But the claim — that industry influence led to the concealing of data — carries echoes, some experts said, of an earlier era of drug marketing, when crucial clinical data went missing from journal articles, leading to high-profile corrections and a wave of ethics policies to limit the influence of drug companies on medical literature…
Last month the Duke researchers published an analysis in The New England Journal of Medicine and concluded that the problems with the device did not change the trial’s results. But some in the medical community questioned their findings because their method required them to essentially guess which groups of patients were more likely to be affected by the malfunctioning device. A better way to evaluate the device, other researchers said, would be to compare the device readings with test results that were done at a central laboratory. Investigators did that at two points in the trial, drawing blood from more than 5,000 of the patients who took warfarin and sending the samples for testing. The blood was taken 12 and 24 weeks after patients enrolled in the trial. But the Duke researchers made no mention of the lab data in their letter. In an interview, journal editors said they did not know about the lab data until last Tuesday, when a reporter for The New York Times asked them about it. “At the time we published the letter, we didn’t know that it existed,” said Dr. Jeffrey M. Drazen, editor in chief of The New England Journal of Medicine…
In a footnote, the lawyers said that during the process of vetting the Duke researchers’ letter, a peer reviewer asked about the existence of lab data that would allow a comparison with the device’s readings. “Despite being provided this opportunity to respond to the peer reviewers,” the lawyers said, the “defendants remained silent on this point, thereby misleading the NEJM.” Dr. Drazen confirmed that a peer reviewer, whose identities are kept confidential, had asked about such data, but said the editors had rephrased the question to ask whether such data was available throughout the course of the trial. Duke then answered no, he said. The letter’s three authors, two from Duke and one affiliated with the University of Edinburgh in Scotland, declined to comment, as did a spokesman for Duke…
Dr. Steven Nissen, a cardiologist at the Cleveland Clinic, served on the Food and Drug Administration advisory panel that voted to approve Xarelto in 2011. He was one of two members who voted against the drug. He expressed doubt that any after-the-fact analysis would give doctors and patients answers. “Given the fact that the device was inaccurate, there is no way anybody can tell you what would have happened in the trial,” he said.
“leading the NEJM.” Dr. Drazen confirmed that a peer reviewer, whose identities are kept confidential, had asked about such data, but said the editors had rephrased the question to ask whether such data was available throughout the course of the trial. Duke then answered no, he said. The letter’s three authors, two from Duke and one affiliated with the University of Edinburgh in Scotland, declined to comment, as did a spokesman for Duke…”
“Oh yeah, and what about that rephrasing in red above?…”
Perhaps:
A journal’s peer review process is only as good as its editor in chief?
A journal’s post-publication commitment to the needs of its clinical readership is only as good as its editor in chief?
A pattern that repeats.
Cue the movie The Fugitive, with Harrison Ford, and one of the main plot elements was the fact that he was going to expose this experimental drug Provasic caused liver damage.
Yeah, just a movie, but, he was going to be killed because he was going to tell the truth.
Art imitates life, they don’t come up with these screenplay ideas out of thin air, and certainly, it won’t result in thin blood…
Don’t underestimate the enemy here, when drugs mean money, money means power, and power means people are expendable…