by Preskorn S, Macaluso M, Mehra DO, Zammit G, Moskal JR, Burch RM, and the GLYX-13 Clinical Study GroupJournal of Psychiatric Practice. 2015 21[2]:140-149.
BACKGROUND: Approximately 45% of patients with major depressive disorder [MDD] do not remit when treated with biogenic amine antidepressants. Consequently, there is a significant need for antidepressant agents with different mechanisms of action. Early proof of concept [POC] studies with such novel agents play a significant role in helping drug developers identify agents and mechanisms of action that merit more intensive research. Studies have demonstrated that high affinity N-methyl-Daspartate [NMDA] receptor blockers [eg, ketamine] can produce rapid antidepressant effects in patients who have not responded to currently available agents, but treatment with these agents is accompanied by psychotomimetic effects that make their use problematic. This column describes a POC study involving GLYX-13, an N-methyl-D-aspartate receptor glycine site functional partial agonist.METHOD: In this double-blind, randomized, placebo-controlled study, a single intravenous [IV] dose of GLYX-13 [1, 5, 10, or 30 mg/kg] or placebo was administered to 116 subjects with MDD who had not benefitted from a trial of at least one biogenic amine antidepressant during the current episode. The primary outcome measure was score on the Hamilton Depression Rating Scale-17 [Ham-D17], which was used to rate overall depressive symptoms at baseline and at 24 hours and days 3, 7, 14, and, in some arms, days 21 and 28 after administration.RESULTS: GLYX-13, 5 or 10 mg/kg IV, reduced depressive symptoms as assessed by the Ham-D17 at days 1 through 7. Onset of action as assessed using the Bech-6 occurred within 2 hours. GLYX-13 did not elicit psychomimetic or other significant side effects.CONCLUSION: In this early POC study, GLYX-13 reduced depressive symptoms within 2 hours and this effect was maintained for 7 days on average in subjects with MDD who had not responded to another antidepressant agent during the current depressive episode. The findings of this study support the hypothesis that modulation of the NMDA receptor is a valid target for the development of antidepressant drugs and the need for additional studies to further evaluate the effects of GLYX-13. POC studies such as the one described here play a pivotal role in allowing drug researchers to decide whether to move forward with larger and more expensive studies, and they enable them to focus available resources on those molecules that appear to have the most therapeutic promise. Based on the POC study described here, a multiple dose study has been completed which showed sustained therapeutic benefit with repeated dosing of GLYX-13 for more than 6 weeks. Phase 3 studies are now being planned.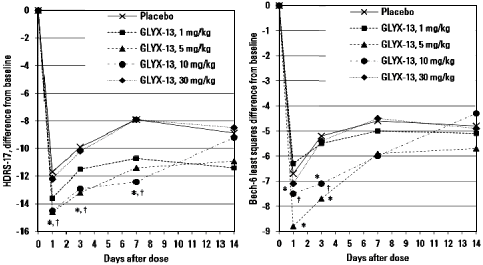
DUBLIN, Jan. 29, 2016 /PRNewswire/ — Allergan plc. [NYSE: AGN], a leading global pharmaceutical company today announced that its Phase III ready investigational medication rapastinel [GLYX-13] received Breakthrough Therapy designation from the U.S. Food and Drug Administration [FDA] for adjunctive treatment of Major Depressive Disorder [MDD]. This follows the Fast Track Designation for rapastinel granted by the FDA in 2014.
"Rapastinel is the first Allergan medicine to be granted Breakthrough Therapy designation by the FDA, underscoring our commitment to innovative research and development that addresses significant unmet medical needs. Breakthrough Therapy designation will allow us to work more closely with the FDA to bring this important therapy to patients as rapidly as possible," said David Nicholson, Executive Vice President and President of Global R&D brands at Allergan. "There remains an unmet medical need for agents in depression that demonstrate a rapid onset of action. We believe that rapastinel has great potential to fulfill that unmet medical need in major depressive disorder."…
The Breakthrough Therapy designation was based on preclinical and preliminary clinical evidence for rapastinel, which supports a rapid [within 1 day] and sustained antidepressant effect over the course of the Phase II studies. Rapastinel has been found to be well tolerated in studies to date, with no psychotomimetic or hallucinogenic side effects observed…
Enacted as part of the 2012 FDA Safety and Innovation Act [FDASIA], Breakthrough Therapy designation is intended to expedite the development and review of a potential new medicine if it is intended to treat a serious or life-threatening disease and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies. The Breakthrough Therapy designation is distinct from FDA’s other mechanisms to expedite drug development and review…
Here are a few quick reactions. First, the published report by Preskorn et al indicates significant protocol changes that were not recorded on ClinicalTrials.gov. These include Bech-6 outcome measure in place of BPRS; dose increase up to 30mg IV. In turn, these raise the possibility of HARKing.
Second, the Figures you showed tell us the placebo effect is in the same ballpark as the drug effect and it is just as enduring. That says a lot about the “treatment resistance” of these patients. We are not talking day versus night here.
Third, there is no clear dose-response pattern in the Figures. Surprisingly, the 30mg dose was no better than placebo.
Fourth, there is a laundry list of drugs that have some effect on depressive symptoms, at least for a while – anticholinergics like benztropine and scopolamine; GABA agonists like benzodiazepines and alcohol; mu-opiate agonists like morphine, etc; cannabinoids; amphetamines; and so on. That is not surprising when we consider the cortico-striato-thalamo-cortical circuitry involved, which is hyperactive in melancholic depression. Any drug whose net effect is to change circuit activity will do something – at least for a while. Now consider that glutamate is the major excitatory transmitter in these mood circuits and it’s no surprise that interfering with glutamate function by ketamine or GLYX-13 seems to do something. But is it a genuine breakthrough? The effect sizes seen in the Figures suggest that it is not. Has the FDA once again been fooled to give GLYX-13 breakthrough status? That’s what it looks like to me.
“Has the FDA once again been fooled to give GLYX-13 breakthrough status? That’s what it looks like to me.”
Yeah, me too. I tried to find some record of the proceedings that lead to the FDA Breakthrough status, but couldn’t find anything. I hoped it might reveal something about that ‘second study.’ The published article is now a year old with no Results posted on Clinical Trials…
Am I the only person who peruses this blog who doesn’t just think, but knows that Charles Nemeroff is an antisocial cloaked in the guise of being an MD?
I don’t know, maybe the pervasive lying, lack of remorse, and being caught doing criminal things are the foundations to the antisocial dx until proven otherwise!?
Are psychiatrists afraid to acknowledge a person in academia can be as disruptive and dangerous as some solo practitioner, like the scumbag in Georgia who was arrested for at least 36 deaths recently? Hey, Dr N, this was in your own state, not obviously related to you, but, doesn’t that hit a bit close to home?
http://www.nydailynews.com/news/national/georgia-doctor-arrested-36-patients-die-article-1.2498832
If a causal relationship to a med Nemeroff preached as the next coming of panacea winded up killing phase three participants, would this blog and the readers finally call for his head???
Yeah, some would if not already have, but, what defines irrefutable outrage?
I’ll give you my definition: Charles Nemeroff and his history the past, what, 20 years!!??
Dr. Hassman, Charles Nemeroff has taken quite a few hits for which I and Robert Rubin will claim most of the credit – along with Paul Thacker who was on Senator Grassley’s staff. Here is a partial listing. As the saying goes, he was taken down a peg or two. More importantly, he is no longer the KOL Boss of Bosses – that’s history.
Dr Carroll: I am sure you have done your best, but, where are the other 79.9% of colleagues who should have the strength and tenacity to marginalize, and the more profoundly ostracize this man so his only refuge by now would be the proverbial “ACME” school of Medicine?
Oh, and I pick 80% as I unfortunately know we will never have 95% of our colleagues rise as a majority collective and get rid of the inappropriate 5% that should be the acceptable percentage of impaired colleagues. But, it should be an 80% group, and even that number is not true at the end of the day.
But, thank you for chiming in as one who does care and tries to “take out the trash” that unfortunately collects in every profession in this culture.
Maybe time to start marginalizing U of Miami’s Psych program as well???
“where are the other 79.9% of colleagues who should have the strength and tenacity to marginalize, and the more profoundly ostracize this man so his only refuge by now would be the proverbial “ACME” school of Medicine?”
Many are still fawning all over him at CME events. And look who’s speaking right before him! It’s the Splash Brothers of KOL-world.
http://www.hms-cme.net/734280/
Here is a peak at a recent protest outside the Copley.
http://patch.com/massachusetts/backbay/leaders-child-psychiatry-protested-crimes-against-humanity
I know there is potential for disparaging this action– based upon the group who organized the protest. Makes me wonder if only those used to being vilified have the courage to wage a pubic awareness campaign? Surely, these public protests are not for the faint hearted. 😉
The elite institutional KOLs are the best unwitting allies that CCHR ever had. If they keep it (overpathologizing everything) up, CCHR will be amassing major political victories over psychiatry in the next twenty years.
CCHR is not just a broken clock here, they are winning the debate with the public slowly but surely.
Institutional psychiatry would be well advised at this juncture to knock it off and focus on the 15-20 major illnesses instead of trying to be everything (or do anything) to everyone.
Amen…
BTW, lying liars gotta lie…followup on Valeant:
http://247wallst.com/healthcare-business/2016/03/15/valeant-pharma-trips-over-preliminary-earnings-and-guidance/
It’s trading at about half of what it was two months ago.
I agree with the above comments. The issue arose when the academics among us elected to shun Pharma trials while at the same time accepting and at times soliciting consulting contracts from Pharma. As a fellow during the early 1990’s and considering a Pharrma career opportunity after fellowship I was advised against Pharma. They were mercenaries, no interest in science, and careers were short. With dwindling federal grant money I joined Pharma. I soon realized that many faculty advisors were consultants to the Pharma industry. Where we were avoiding them, and could offer our recent clinical science training to their R&D programs, we saw academics gobbling up piles money, enjoying sales and marketing excursions, sharing R&D secrets across companies, and basically cashing in while non or underperforming back at the university…can’t be in two places at the same time!. Soon the party extended to these same individuals asking for the clinical trials to be placed at their academic centers while simultaneously stating that no private research sites should even do clinical trials. Getting even bolder a few opened private research clinics nearby their academic offices. Talk about calling the kettle black!
So why did no one stand up. a few did, and in an attempt to protect their life with the golden goose they ganged up and took them down one by one in order to make an example of them and to scare off others. You know it worked. Many a career were adversely affected because of what became known within the Pharma industry as the “psychiatric mafia”. We saw them, tried to avoid having to use them as advisors and as a group across competing Pharma companies talked in secret (in order to help our peers) of what things to watch for and to avoid. In our midst we had Stanford, Yale, Wisconsin, Penn, and the “Chairman of the Board” Emory.
The Boston Globe did an expose, the New York Times, Wall Street Journal, etc. but interestingly local newspapers provided no coverage of these AP stories…not until a certain Senator started his investigator with Mr. Thacker at the helm. only then did the paper in Atlanta start running stories, but by then the damage to great drugs and their development plans, financial gluttony, abuse of power and trust within academia and within the public eye was completed.
Has it stopped? NO! HELL NO! Today we have an academic creep back in as advisors. we even have universities starting academic-private companies selling new rating scales, new technologies at patient assessments, external rating companies, and more contracting with Pharma and in so doing they “GET” a trial offered to the university. How often do you see a new drug with a first journal article of a pilot trial with no academic faculty as authors? What is the role of the academic investigator? NIH funded research OR Pharma funded research? Let them focus on Investigator Initiated Trials and NIH funded trails and let Pharma work with the professionals that know and follow FDA regulations, see patients each and every day, and have no ulterior motives other than do their work as clinicians.
If we allow them to form their cliques again then the gang-rape of Pharma and the gang attacks on whistleblowers will start all over again.