- A word to the wise about ketamine
by Alan Schatzberg
AJP 2014 171:262-264.
March 2014. - The ketamine challenge: when practice leaps ahead of science
by Jeffrey Lieberman
PsychiatricNews
February 3, 2015. - Ketamine and Other NMDA Antagonists:
Early Clinical Trials and Possible Mechanisms in Depression
by Jeffrey Newport, Linda Carpenter, William McDonald, James Potash, Mauricio Tohen, Charles Nemeroff, and The APA Council of Research Task Force on Novel Biomarkers and Treatments
AJP 2015 172:950-966.
October 2015.
by Jaskaran B. Singh , Maggie Fedgchin, Ella Daly, Liwen Xi, Caroline Melman, Geert De Bruecker, Andre Tadic, Pascal Sienaert, Frank Wiegand, Husseini Manji, Wayne C. Drevets, and Luc Van NuetenBiological Psychiatry. November 3, 2015. EPub ahead of print.
BACKGROUND: The purpose of this study was to assess the efficacy and safety and to explore the dose response of esketamine intravenous [IV] infusion in patients with treatment-resistant depression [TRD].METHODS: This multicenter, randomized, placebo-controlled trial was conducted in 30 patients with TRD. Patients were randomly assigned 1:1:1 to receive an IV infusion of .20 mg/kg or .40 mg/kg esketamine or placebo over 40 minutes on day 1. The primary end point was change in Montgomery-Åsberg Depression Rating Scale total score from day 1 [baseline] to day 2. Nonresponders who received placebo on day 1 were randomly assigned again 1:1 to IV esketamine .20 mg/kg or .40 mg/kg on day 4. Secondary efficacy and safety measures were also evaluated.RESULTS: Of the enrolled patients, 97% [29 of 30] completed the study. The least squares mean changes [SE] from baseline to day 2 in Montgomery-Åsberg Depression Rating Scale total score for the esketamine .20 mg/kg and .40 mg/kg dose groups were -16.8 [3.00] and -16.9 [2.61], respectively, and showed significant improvement [one-sided p = .001 for both groups] compared with placebo [-3.8 [2.97]]. Esketamine showed a rapid [within 2 hours] and robust antidepressant effect. Treatment-emergent adverse events were dose dependent. The most common treatment-emergent adverse events were headache, nausea, and dissociation; the last-mentioned was transient and did not persist beyond 4 hours from the start of the esketamine infusion.CONCLUSIONS: A rapid onset of robust antidepressant effects was observed in patients with TRD after a 40-min IV infusion of either .20 mg/kg or .40 mg/kg of esketamine. The lower dose may allow for better tolerability while maintaining efficacy
Johnson and Johnson: Press ReleaseAugust 16, 2016Janssen Research & Development, LLC, one of the Janssen Pharmaceutical Companies of Johnson & Johnson, announced today that the U.S. Food and Drug Administration [FDA] has granted a Breakthrough Therapy Designation for esketamine, an investigational antidepressant medication, for the indication of major depressive disorder with imminent risk for suicide. If approved by the FDA, esketamine would be one of the first new approaches to treat major depressive disorder available to patients in the last 50 years.
This also marks the second time esketamine has received a Breakthrough Therapy Designation from the U.S. regulatory authority. Esketamine was first granted this designation for treatment-resistant depression in November 2013. Breakthrough Therapy Designation is intended to expedite development and review timelines when preliminary clinical evidence indicates the drug may demonstrate substantial improvement on one or more clinically significant endpoints over available therapies for serious or life-threatening conditions.
The esketamine Phase 2 clinical trial data presented by Janssen in May 2016 at the Society of Biological Psychiatry 71st Annual Scientific Meeting in Atlanta, Georgia, provided preliminary clinical evidence to support the Breakthrough Therapy Designation for major depressive disorder with imminent risk for suicide.
About EsketamineEsketamine for intranasal administration is an investigational compound being studied by Janssen as part of a global development program. Esketamine is a non-competitive and subtype non-selective activity-dependent N-methyl-D-aspartate [NMDA] receptor antagonist, which has a novel mechanism of action, meaning it works differently than currently available therapies for depression. The program in treatment-resistant depression is currently in Phase 3, with six ongoing clinical trials…[see also Party drug ketamine closer to approval for depression – CNN]

 I’ll have to admit that the notion of seeing a suicidal patient and offering them a sniff of some version of Special K as a treatment strikes me as borderline ludicrous, hard for me to say with a straight face – but stranger things have happened. That’s not why I’m writing about it. Remember, this is Johnson and Johnson, the people who gave us TMAP and all of the Risperidal® sheenanigans. The CEO is still Alex Gorsky – West Point graduate and lifelong Boy Scout, who brought in Billions with all kinds of hanky-panky, flooding the literature with articles churned out by Excerpta Medica. Johnson and Johnson gladly paid the cost-of-doing-business fines of around $2.2 B – trivial in the face of their ill-gotten gains. And with Risperidal®, they were also first-on-the-market:
I’ll have to admit that the notion of seeing a suicidal patient and offering them a sniff of some version of Special K as a treatment strikes me as borderline ludicrous, hard for me to say with a straight face – but stranger things have happened. That’s not why I’m writing about it. Remember, this is Johnson and Johnson, the people who gave us TMAP and all of the Risperidal® sheenanigans. The CEO is still Alex Gorsky – West Point graduate and lifelong Boy Scout, who brought in Billions with all kinds of hanky-panky, flooding the literature with articles churned out by Excerpta Medica. Johnson and Johnson gladly paid the cost-of-doing-business fines of around $2.2 B – trivial in the face of their ill-gotten gains. And with Risperidal®, they were also first-on-the-market: 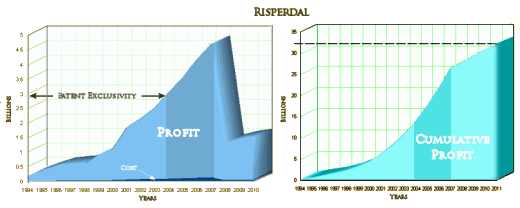
Here’s a roster of their Esketamine clinical trials from clinicaltrials.gov. As you can see, they’re going long on Esketamine. It’s a hungry market and they’re going after it. And then there’s that old saying, "The best predictor of future behavior is past behavior."
| NCT # |
Title | Status | Phase | Start | ||
| NCT01394757 | Network Dysfunction, Schizophrenia and Pharmacological Magnetic Resonance Imaging [phMRI] | Completed | – | 08/2011 | ||
| NCT00847418 | Pharmacokinetics and Pharmacodynamics of Nasally Applied Esketamine | Completed | 1 | 02/2009 | ||
| NCT01780259 | A Study to Assess the Pharmacokinetics, Safety, and Tolerability of Intranasally Administered Esketamine in Healthy Participants | Completed | 1 | 12/2012 | ||
| NCT02060929 | A Study to Evaluate the Pharmacokinetics of Intranasal Esketamine Administered With and Without a Nasal Guide on the Intranasal Device | Completed | 1 | 10/2013 | ||
| NCT01980303 | A Study to Assess the Pharmacokinetics of Intranasally Administered Esketamine in Healthy Japanese and Caucasian Volunteers | Completed | 1 | 11/2013 | ||
| NCT02129088 | A Pharmacokinetic, Safety and Tolerability Study of Esketamine in Healthy Elderly and Adult Participants | Completed | 1 | 03/2014 | ||
| NCT02094378 | A Study to Evaluate the Effect of Intranasal Esketamine on Cognitive Functioning in Healthy Subjects | Completed | 1 | 06/2014 | ||
| NCT02154334 | Study to Assess the Effects of Allergic Rhinitis and Co-administration of Mometasone or Oxymetazoline on the Pharmacokinetics, Safety, and Tolerability of Intranasal Esketamine | Completed | 1 | 06/2014 | ||
| NCT02228239 | Study to Assess the Effects of Esketamine on Safety of On-road Driving in Healthy Participants | Completed | 1 | 09/2014 | ||
| NCT02345148 | Pharmacokinetic, Safety, and Tolerability Study of Intranasally Administered Esketamine in Elderly and and Healthy Younger Adult Participants | Completed | 1 | 12/2014 | ||
| NCT02343289 | A Study to Evaluate the Absolute Bioavailability of Intranasal and Oral Esketamine and the Effects of Clarithromycin on the Pharmacokinetics of Intranasal Esketamine in Healthy Participants | Completed | 1 | 01/2015 | ||
| NCT02568176 | Pharmacokinetic Study of Intranasal Esketamine and Its Effects on the Pharmacokinetics of Orally-Administered Midazolam and Bupropion in Healthy Participants | Completed | 1 | 10/2015 | ||
| NCT02611505 | A Study to Assess the Effects of Hepatic Impairment on the Pharmacokinetics, Safety, and Tolerability of Intranasally Administered Esketamine | Recruiting | 1 | 11/2015 | ||
| NCT02606084 | A Study to Assess the Effects of Renal Impairment on the Pharmacokinetics, Safety, and Tolerability of Intranasally Administered Esketamine | Recruiting | 1 | 12/2015 | ||
| NCT02846519 | Pharmacokinetic, Safety, and Tolerability Study of Intranasally Administered Esketamine in Healthy Han Chinese, Korean, Japanese, and Caucasian Participants and the Effects of Rifampin on the Pharmacokinetics of Intranasally Administered Esketamine | Completed | 1 | 02/2016 | ||
| NCT02682225 | Crossover Study to Evaluate the Abuse Potential of Intranasal Esketamine Compared to Racemic Intravenous Ketamine in Nondependent, Recreational Drug Users | Recruiting | 1 | 03/2016 | ||
| NCT02674295 | A Mass Balance Study With a Microtracer Dose of 14C-esketamine in Healthy Male Participants | Recruiting | 1 | 03/2016 | ||
| NCT02737605 | A Study to Evaluate the Effects of Esketamine on Cardiac Repolarization in Healthy Participants | Not yet recruiting | 1 | 07/2016 | ||
| NCT02857777 | Pharmacokinetic, Safety, and Tolerability Study of Intranasally Administered Esketamine in Elderly Japanese, and Healthy Younger Adult Japanese Subjects | Not yet recruiting | 1 | 08/2016 | ||
| NCT01640080 | A Study of the Efficacy of Intravenous Esketamine in Adult Patients With Treatment-Resistant Depression | Completed | 2 | 06/2012 | ||
| NCT01998958 | A Study to Evaluate the Safety and Efficacy of Intranasal Esketamine in Treatment-resistant Depression | Completed | 2 | 01/2014 | ||
| NCT02133001 | A Double-blind Study to Assess the Efficacy and Safety of Intranasal Esketamine for the Rapid Reduction of the Symptoms of Major Depressive Disorder, Including Suicidal Ideation, in Participants Who Are Assessed to be at Imminent Risk for Suicide | Completed | 2 | 05/2014 | ||
| NCT02417064 | A Study to Evaluate the Efficacy, Safety, and Tolerability of Fixed Doses of Intranasal Esketamine Plus an Oral Antidepressant in Adult Participants With Treatment-resistant Depression | Recruiting | 3 | 08/2015 | ||
| NCT02418585 | A Study to Evaluate the Efficacy, Safety, and Tolerability of Flexible Doses of Intranasal Esketamine Plus an Oral Antidepressant in Adult Participants With Treatment-resistant Depression | Recruiting | 3 | 08/2015 | ||
| NCT02422186 | A Study to Evaluate the Efficacy, Safety, and Tolerability of Intranasal Esketamine Plus an Oral Antidepressant in Elderly Participants With Treatment-resistant Depression | Recruiting | 3 | 08/2015 | ||
| NCT02497287 | A Long-term, Safety and Efficacy Study of Intranasal Esketamine in Treatment-resistant Depression | Recruiting | 3 | 09/2015 | ||
| NCT02493868 | A Study of Intranasal Esketamine Plus an Oral Antidepressant for Relapse Prevention in Adult Participants With Treatment-resistant Depression | Recruiting | 3 | 10/2015 | ||
| NCT02782104 | A Long-term Safety Study of Intranasal Esketamine in Treatment-resistant Depression | Recruiting | 3 | 06/2016 |
It appears that the metabolite of ketamine is what produces the clinical effects and its mechanism is different from the parent drug:
http://slatestarcodex.com/2016/06/07/ketamine-research-in-a-new-light/
As an addiction psychiatrist, my concern is the cultural swings from permissiveness to prohibition when it comes to addictive drugs as suggested by Musto in the past. There is hardly a week that goes by where there is not a headline about the therapeutic aspects of hallucinogens or cannabis typically based on scant data. The article from Nature earlier this year highlights how deficient a lot of this research may be.
http://real-psychiatry.blogspot.com/2016/05/latest-on-ketamine.html
Always enjoy neuroskeptic posts, in both style and substance:
http://blogs.discovermagazine.com/neuroskeptic/2016/05/07/ketamine-depression-breakthrough/#.V7dMfVRHbCQ
1boringyoungman-
Do you (or others) know what “a pronounced psychological response to it psychoactive effects” mean? This is from the neuroskeptic article.
I think we struggle still with not wanting to see our drugs as the same as the street drugs.
Yet ect and maois are out of the arsenal in most practices…
I sincerely do hope this pans out though
Dr. Steingard, I think this link might address at least part of your question?
http://blogs.discovermagazine.com/neuroskeptic/2014/02/08/depression-ketamine-struck-gold/#.V7fSzVRHbCQ
It remains to be clarified what mechanism(s) are at play in Ketamine’s potential effects on depression. But it seems worth keeping in mind when “the nullification of another urban drug legend” is posited, that the Nature paper, though impressive, was entirely with rodents.
Dr. Steingard, would also recommend reading the link kindly provided by Mitchell at the top of this thread.
Ella Daly, who coauthored the paper on the use of intravenous esketamine for so-called treatment-resistant depression, was a member of Madhukar Trivedi’s inner circle at UT Southwestern during TMAP’s heyday. When Senator Grassley started asking questions, and the Texas Medicaid fraud trial was on the horizon, Daly found a home at Johnson and Johnson (Janssen).
Texas v. Janssen LP, D-1GV-04-001288, District Court, Travis County, Texas (Austin)
1bom, As an entirely different aside: in reading back over an earlier related post I came across an old comment I had missed. Fascinating:
http://1boringoldman.com/index.php/2016/03/10/shame-on-us/#comment-263730
Huh.
All the flap and fuss over the latest drug (whether esketamine or something else) misses an extremely important body of research. And that is the role that Adverse Childhood Experiences and other psychological injuries play in the development of depression. The research from Drs. Felitti and Anda and the CDC showed a perfect dose response relationship, that the more types of childhood trauma a person experienced, the higher the likelihood of a person developing depression in their lifetime. Women with no ACE had an 18% chance of developing depression, whereas women with 6 or more ACE’s had a 61% chance of developing depression by their mid-fifties. Very similar results were obtained in Canada in a stratified random sample of 22,300 Canadians, published a couple years ago in the Canadian Medical Association Journal.
I agree we need to carefully monitor the shenanigans of PHARMA, and think IBOM does an excellent job. We also need to point out what actually is the single largest cause of depression, and educate the public about the value of psychotherapy in healing those psychological injuries, rather than reflexively prescribing a pill.
I have treated a large number of patient with ketamine. Second patient I ever treated was in maintenance ECT and had received well over 50 ECT. Ketamine for that patient was curative, a true miracle, over night elimination of the depressive symptoms. That being said in my experience about 1/3 have dramatic sudden benefit from ketamine, 1/3 have a good but not over whelming benefit and 1/3 have no real benefit. For those patient that are strong responders, the benefit is quite remarkable, with remission after one or a very few treatments. This is not due to them being high or some lasting euphoric effect from intoxication, but a true, rapid “antidepressant” response, that lasts from 1 day to weeks or months depending on the patient. And therein lies the problem, the response for most patients last only weeks, so they end up in regular maintenance therapy.
Thanks for that comment, Dr. Garlow. There is indeed a place for experimental therapeutics, and sometimes there are positive surprises. It’s just too bad that experimental therapeutics has been mostly hijacked by corporate interests and academic wannabe entrepreneurs.