Nosing around about Ketamine, I ran across this draft that I never posted from 2½ years ago:
|
from February 19, 2014: Neuroskeptic has an interesting thing going trying to figure out if the effect on depressed people from Ketamine is because Ketamine is a pharmacologic antidepressant, or, to put it in my own worlds, does getting
 That’s going to be a hard comparator to find! And it begs the question if it’s helping because it gets the depressed "out of themselves." If you’ve worked an ER where people have been brought having taken Ketamine, it’s just hard to imagine using it as a therapeutic agent. They are way "out of themselves!" So here’s what Neuroskeptic said:
 In summary I think the question is still very much open whether ketamine’s antidepressant effects are ‘psychological’ or ‘physiological’ in origin. I’m genuinely agnostic on the issue; I would love to know the answer, but at present we don’t have it. In summary I think the question is still very much open whether ketamine’s antidepressant effects are ‘psychological’ or ‘physiological’ in origin. I’m genuinely agnostic on the issue; I would love to know the answer, but at present we don’t have it.I’m going to vote ‘psychological’…
|
I’m obviously not quite so agnostic as Neuroskeptic when it comes to Ketamine, but I’m open to being born·again given something really solid. The question remains, "Is the manifest improvement in depressive symptoms following the initial psychodelic experience ‘psychological’ or ‘physiological’?" and I have to concede that I don’t really know the answer. I certainly can’t argue that there’s not an effect [this Janssen article from the recent AJP]:
by Jaskaran B. Singh, Maggie Fedgchin, Ella J. Daly, Peter De Boer, Kimberly Cooper, Pilar Lim, Christine Pinter, James W. Murrough, Gerard Sanacora, Richard C. Shelton, Benji Kurian, Andrew Winokur, Maurizio Fava, Husseini Manji, Wayne C. Drevets, and Luc Van Nueten«Janssen Employees, Janssen COI»American Journal of Psychiatry. 2016 173:816–826.
Objective: Ketamine, an N-methyl-D-aspartate glutamate receptor antagonist, has demonstrated a rapid-onset antidepressant effect in patients with treatment-resistant depression. This study evaluated the efficacy of twice- and thrice-weekly intravenous administration of ketamine in sustaining initial antidepressant effects in patients with treatment-resistant depression.Method: In a multicenter, double-blind study, adults [ages 18–64 years] with treatment-resistant depression were randomized to receive either intravenous ketamine [0.5 mg/kg of body weight] or intravenous placebo, administered over 40 minutes, either two or three times weekly, for up to 4 weeks. Patients who discontinued double-blind treatment after at least 2 weeks for lack of efficacy could enter an optional 2-week open-label phase to receive ketamine with the same frequency as in the double-blind phase. The primary outcome measure was change from baseline to day 15 in total score on the Montgomery-Åsberg Depression Rating Scale [MADRS].Results: In total, 67 [45 women] of 68 randomized patients received treatment. In the twice-weekly dosing groups, the mean change in MADRS score at day 15 was -18.4 [SD=12.0] for ketamine and -5.7 [SD=10.2] for placebo; in the thrice-weekly groups, it was -17.7 [SD=7.3] for ketamine and -3.1 [SD=5.7] for placebo. Similar observations were noted for ketamine during the open-label phase [twice-weekly, -12.2 [SD=12.8] on day 4; thrice-weekly, -14.0 [SD=12.5] on day 5]. Both regimens were generally well tolerated. Headache, anxiety, dissociation, nausea, and dizziness were the most common [>20%] treatment-emergent adverse events. Dissociative symptoms occurred transiently and attenuated with repeated dosing.Conclusions: Twice-weekly and thrice-weekly administration of ketamine at 0.5 mg/kg similarly maintained antidepressant efficacy over 15 days.
by Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA Jr, Gould TDNature. 2016 533[7604]:481-486.
Major depressive disorder affects around 16 per cent of the world population at some point in their lives. Despite the availability of numerous monoaminergic-based antidepressants, most patients require several weeks, if not months, to respond to these treatments, and many patients never attain sustained remission of their symptoms. The non-competitive, glutamatergic NMDAR [N-methyl-d-aspartate receptor] antagonist [R,S]-ketamine exerts rapid and sustained antidepressant effects after a single dose in patients with depression, but its use is associated with undesirable side effects. Here we show that the metabolism of [R,S]-ketamine to [2S,6S;2R,6R]-hydroxynorketamine [HNK] is essential for its antidepressant effects, and that the [2R,6R]-HNK enantiomer exerts behavioural, electroencephalographic, electrophysiological and cellular antidepressant-related actions in mice. These antidepressant actions are independent of NMDAR inhibition but involve early and sustained activation of AMPARs [α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors]. We also establish that [2R,6R]-HNK lacks ketamine-related side effects. Our data implicate a novel mechanism underlying the antidepressant properties of [R,S]-ketamine and have relevance for the development of next-generation, rapid-acting antidepressants.
Discover MagazineBy NeuroskepticMay 7, 2016In recent years there has been great research interest in ketamine as an antidepressant. Ketamine, a drug better-known for its use as an anaesthetic [and a recreational drug in lower doses] is claimed to have powerful, rapid-acting antidepressant effects, even in depressed patients who have not responded to more conventional drugs. However, its mechanism of action remains unclear.
Now, in a major new Nature paper, Baltimore researchers Panos Zanos and colleagues say that ketamine itself is probably not an antidepressant after all. Instead, the antidepressant effects can be attributed to a metabolite of the drug, formed in the body after taking ketamine. The metabolite is called [2S,6S;2R,6R]-HNK. But is this a real breakthrough or a red herring? Here’s how Zanos et al. describe their findings:
Here we show that the metabolism of [R,S]-ketamine to [2S,6S;2R,6R]-hydroxynorketamine [HNK] is essential for its antidepressant effects, and that the [2R,6R]-HNK enantiomer exerts behavioural, electroencephalo-graphic, electrophysiological and cellular antidepressant-related actions in mice.So how does HNK do this? Unlike ketamine, HNK is not an NMDA glutamate receptor antagonist. Rather, it acts to promote signalling via the AMPA glutamate receptor. NMDA antagonism is believed to underlie ketamine’s anaesthetic and psychoactive [dissociative hallucinogenic] properties. So HNK is not likely to have these undesirable effects, Zanos et al. say, making it more suitable as an antidepressant.
I’ve been following the ketamine/depression story for nearly a decade and this paper is the most interesting piece of work I’ve seen. This is a really impressive set of experiments – but they were all in animals. Zanos et al. haven’t shown that ketamine’s antidepressant effects are mediated by HNK in people; rather they’ve studied the ‘antidepressant-like‘ effects of ketamine in rodent behavioural models such as the forced swim test. Bottom line: we don’t yet know whether HNK is a human antidepressant.
My own suspicion is that the antidepressant effects of ketamine may be driven not by a metabolite but as a kind of psychological reaction to its pronounced psychoactive effects. If HNK is non-psychoactive, I predict that it won’t turn out to mediate ketamine’s antidepressant effects in humans. HNK might nonetheless have some antidepressant activity but not as dramatic as ketamine. Then again I might very well be wrong, and I hope I am, because if Zanos et al. are right, they might just have discovered the next ‘miracle’ antidepressant.
- Real Psychiatry: Latest on Ketamine
- Slate Star Codex: Ketamine Research In A New Light
And Janssen is certainly going forward full bore with Esketamine intravenous and intranasal [see the best predictor… and Esketamine Receives Breakthrough Therapy Designation from U.S. Food and Drug Administration for Major Depressive Disorder with Imminent Risk for Suicide]. When I looked at their recent study earlier [Intravenous Esketamine in Adult Treatment-Resistant Depression: A Double-Blind, Double-Randomization, Placebo-Controlled Study], I focused on efficacy and didn’t pay enough attention to the club drug effect. They assessed it with both the Brief Psychiatric Rating Scale [BPRS] total score and the Clinician Administered Dissociative States Scale [CADSS] total score [which roughly paralleled each other]. The upper figure is the BPRS data from the paper and the lower versions are redrawn with an accurate x-axis timeline [because I am suspicious of irregular scales in graphic presentations]. It looks like about a 2 – 4 hour "high":
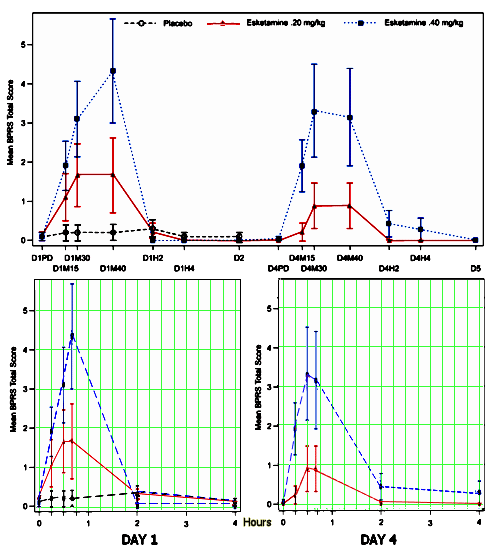
"Similar to ketamine, esketamine led to transient dissociative and psychotic symptoms. According to CADSS severity categories, the peak mean CADSS total scores at 40 minutes postinfusion on day 1 for the esketamine .20 mg/kg and .40 mg/kg groups would be categorized as high. However, symptoms subsided to baseline levels within 4 hours. The CADSS scores showed evidence of dose dependence on each DB dosing day. A similar pattern was observed for psychotic-like effects measured using BPRS total scores. These results are similar to results of IV ketamine studies."
The intranasal form [study not yet published that I can find – reference: Canuso C, et al. "Esketamine for the Rapid Reduction of the Symptoms of Major Depressive Disorder, Including Suicidal Ideation, in Subjects Assessed to be at Imminent Risk for Suicide." Society of Biological Psychiatry 71st Annual Scientific Meeting. May 12-14, 2016] may be an improvement in terms of a delivery system, but it’s not apparent to me how Esketamine makes any advances over the already available racemic mixture of Ketamine in terms of either efficacy or absence of club drug effects.
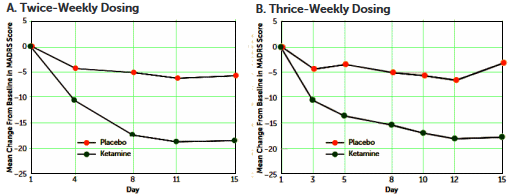
In an era of instant gratification and patient satisfaction surveys, I predict it will take off and become mainstream soon.
“But beyond that, I think of Ketamine as a feel good drug rather than an antidepressant, adding a good feeling instead of ameliorating the target emotion – depression. I question whether this is a rational direction for psychiatric treatment to pursue.”
There is a lot packed into that paragraph. You describe “depression” as an “emotion.” Which I find puzzling. There would appear to be a long history of people, under the right conditions, finding drug related altered experiences to be transformative. Is this really less rational a direction than was ECT? Something given briefly is identified on the ground as then having profound effects on intractable Depression in some individuals, even if the bulk of the effect may last only a few months, and that sparks a direction in treatment efforts.
To my mind it is adding Ketamine into the current culture of academic/journal/industrial/managed psychiatry that produces a toxic brew. MDD is seen as a cash cow. You don’t make $ off the 1% market (well, unless you charge a massive amount per treatment which does work for some companies these days). The model for researchers these days is Charney not Carroll.
Though the destruction that has accompanied Purdue’s oxytocin crusade is cautionary, it almost concerns me more that Charney is one of the biggest players in this game.
The voices of those coming from a more clinical bent, as well as the voices of some who appear to have found notable relief from what sounds like the torment of melancholia (rather than a “instant gratification” desire), makes me hesitate to dismiss the effect of Ketamine on Depression in some.
We rarely hear those seeking rapid relief from Melancholia through ECT as seeking instant gratification.
I completely favor caution. Fool me once,…etc. But would hesitate on dismissal. And, again, found the “depression” itself described as an “emotion” struck me.
Sorry, meant to say: We rarely hear those seeking rapid relief from Melancholia through ECT described as seeking instant gratification.
I’ll respond to the ‘depression as emotion’ part later, but as to dismissing Ketamine. If I implied that, I didn’t mean to. I’m skeptical sure enough…
What I’m balking about is the “cousins” [Rapestinel, Esketamine]. From what I see, that “fast track” runs through the intersection of South Speculation Street and East Entrepreneurial Lane in downtown Pharmaville.
Fair. Thanks.
Ketamine will become commonplace for the same reason Suboxone did…an irresistible narrative
Of Mice and Men…was that only referring to the rat/human studies or a literary double entendre? if so…who is the mouse that gets crushed by Lenny in this scenario?
Maybe even more than “double”…
Is “Lenny” the academic-KOL-publishing complex?
https://www.youtube.com/watch?v=ArNz8U7tgU4
From the Zanos study:
“…Here we show that the metabolism of [R,S]-ketamine to [2S,6S;2R,6R]-hydroxynorketamine [HNK] is essential for its antidepressant effects, and that the [2R,6R]-HNK enantiomer exerts behavioural, electroencephalographic, electrophysiological and cellular antidepressant-related actions in mice. ”
If this study is really on to something, then S-ketamine should be a DUD, since it’s the R-enantiomer that’s doing all the work here. What we need (appears to me that we need anyhow) is a clinical trial comparing…
[2R,6R]-HNK vs. S-ketamine
My only quibble– and it really is nothing more than that– with Neuroskeptic is his reference to seeing people in the ER who have taken Ketamine. Even if they have tested positive for Ketamine in a tox screen, there’s no telling what else they took– and if they didn’t take a tox screen, maybe they were taking MXE, diphenidine or tiletamine (which, I am told, is a truly evil thing to do).
The “party drug” landscape is getting pretty weird. And don’t even get me started on the Shulgin junk– the whole ghastly 2C family and bromodragonfly, etc.
@ 1boringyoungman: Agreed, few drugs seem to have an enduring transformative effect for months, and it’s not a completely useless avenue of inquiry, though here I think it could be sensibly argued that as with antidepressants, the only worthwhile drugs were discovered a long time ago. It’s very frustrating that most of the LSD research with human subjects was done with doses of 100 micrograms or more; one wonders if it would be possible to use a 30 microgram doses, perhaps at intervals as great as one or two years, under controlled therapeutic circumstances. Or even just once. But if you’re thinking of that kind of treatment protocol, it’s hard to think of a sensible reason for messing around with a drug like ketamine.
And I was extremely amused by certain aspects of the oxytocin craze as well, particularly some of the bizarre ways researchers were trying to work around the fact that the half life is about four minutes when administered nasally.
My own experiments with oxytocin were conducted in group therapy with about 20 really warm empathic people, and I found oxytocin to be very effective for relieving depression.
I also liked the fact that the drug was manufactured in my own body– no pills or nasal sprays or worries about contamination, except from my other neurotransmitters.
“My own experiments with oxytocin were conducted in group therapy with about 20 really warm empathic people, and I found oxytocin to be very effective for relieving depression.”
This is one reason I find MDMA research more interesting than ketamine research.