Today is an anniversary for me. This post from a year ago today …
Posted on Wednesday 16 September 2015Well, our RIAT article, Restoring Study 329: efficacy and harms of paroxetine and imipramine in treatment of major depression in adolescence, is finally published online at the British Medical Journal. It’s fairly straightforward. The emphasis is on the harms analysis for obvious reasons – an accurate representation of a drug’s safety is always the first order of business…
|
… marked the end of an intense couple of years, all focused on our RIAT team getting to say this in print:
by Le Noury J, Nardo J, Healy D, Jureidini J, Raven M, Tufanaru C, & Abi-Jaoude E.British Medical Journal. 2015 …
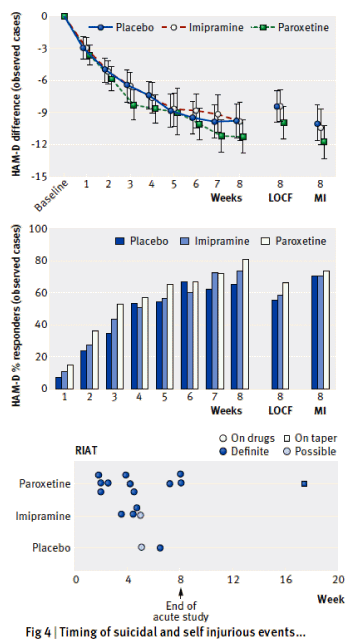
Conclusions: Neither paroxetine nor high-dose imipramine demonstrated efficacy for major depression in adolescents, and there was an increase in harms with both drugs. Access to primary data from trials has important implications for both clinical practice and research, including that published conclusions about efficacy and safety should not be read as authoritative. The reanalysis of Study 329 illustrates the necessity of making primary trial data available to increase the rigour of the evidence base.
There are layers and layers of stories to tell about the writing of this article. It’s a story I expect will ultimately be told in detail. This is just an outline. We had originally planned to reanalyze the data already released by court order strictly following the a priori protocol [from before the study began]. We were able to stick with that plan in the efficacy analysis, supplementing it with a more modern correction method for missing values requested by the peer reviewers [Multiple Imputation]. But that wasn’t possible with the harms analysis. The method for cataloging harms wasn’t specified in the protocol, and the one used in the original paper obscured the findings. So we apploed to GSK for the CRFs [original case report forms] and after another saga in its own right, we were granted remote access [50,000± pages]. The process of gaining the access and using the constricting remote access system are some of those other stories – partially documented on our web site [study329.org].
When it was finally completed and submitted, it wasn’t an ending, but the beginning of another story. Over the next year, it went through seven major resubmissions, multiple peer reviews and independent analyses. Acceptance was never assured until near the end of the process. And there were plenty of frayed nerves within and among everyone involved, particularly near the end of that year. It was something new for the authors, the journal, and the genre – and that showed in the process. I only wish other clinical trial reports were as closely looked at as this one. So the prepublication year was another whole story unto itself.
In an area where people question how involved the listed authors are in the production of a published article, or whether the journals are thorough in their review, our paper stands on its own. It was unfunded research. The authors on the byline were the only act in town. We did the negotiating, extracted the data, ran the analyses, checked each others work, wrote and edited the narrative, drew the graphics, made the submission, fielded the correspondence, etc. No ghosts anywhere in [or out of] sight. As mentioned, it was vetted like none other by the BMJ. And the final paper was well received and is widely quoted. It was definitely all well worth it. I got to work with some amazing colleagues on something that mattered. Who could ask for more than that? I hope others will follow the principles of a RIAT anaysis and take a look at many other questioned studies.
It looks like it took a staggering amount of work to find the errors and distortions in the publication. I note that it was unpaid work, and feel indebted that you and others exerted yourselves to such a degree.
If only there was as much funding and vigor put into correcting the omissions and distortions in the field of psychiatry, as went into creating them in the first place.