While it’s discussed almost like it’s some complex governmental agency, clinicaltrials.gov is just a very large, online, searchable database of clinical trials using human subjects. It’s only a registry, not unlike the bridal registries filled out by prospective brides as the big day approaches…
It may seem like in describing the hit-or-miss quality of this site and its sometimes chaotic organization, I’m complaining. Quite the contrary. I’m mentioning it for several reasons. First, my impression is that it’s not often visited, being the haunt of people who do systematic reviews rather than mere mortals. But mainly I want to say that after five or six years of repeatedly looking up things here, I’m impressed. These reviews are extensive. They’re all different in that the reviewers "follow their noses" going through the data. Sometimes they reanalyze the raw data, slicing and dicing it in different ways from the way it’s submitted. Sometimes they explore different analytic methods. But I’ve never found one where the didn’t follow the a priori protocol or took the sponsor’s version and computation for granted. Their standard is low [two positive studies], but they go at it competently. The statistical reviews are often illuminating. I’ve disagreed with their conclusions at times, but never their methodology or thoroughness. These reports are reviewed by committees who say yeah or nay. Actually, so do the reviewers. And I think the final decision rests with the director of the FDA.
They’re obviously in no hurry to post their documents or to be comprehensive [did I mention swiss cheese], but that’s not what they’re for. And I’ve been glad for what I could get. I’m talking about this for a specific reason. The intended public interface for clinical trials is clinicaltrials.gov, an NIH database created to describe the trial design, its progress, and its results. But it has been purposefully underpopulated and essentially rendered impotent. The real information is inside the FDA with its ability to see all the information [see Evidence of Clinical Effectiveness and Data Requirements For an NDA], but it’s latecoming to their site and spotty for the likes of those of us who try to investigate these drugs and vet the published studies.
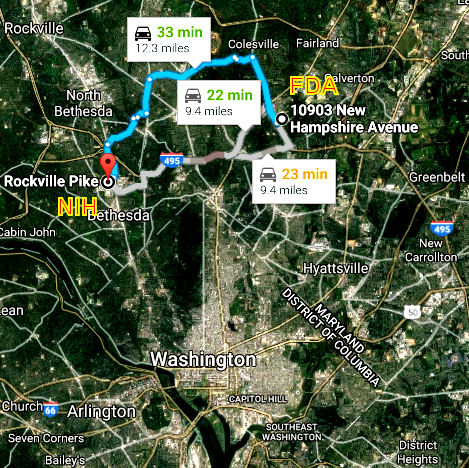
Two windows into the Clinical Trials results just across town from each other, but in some ways, they might as well be on the opposite poles of the planet:
Sorry, the comment form is closed at this time.