by Marc A. RodwinJournal of Health Care Law & Policy. 2015 18:45-84.
A. The Origins of Contemporary Pharmaceutical Regulation
Before examining the oversight of clinical trials, let us briefly review the evolution of the pharmaceutical industry over the last century. In the beginning of the twentieth century, the drug market was premised on the doctrine of laissez-faire. Manufacturers did not have to test their drugs or disclose the ingredients, could make any therapeutic claim, and could sell any product directly to consumers.
Reformers and muckrakers—supported by the American Medical Association [“AMA”]—spearheaded the fight for federal drug regulations. In 1906. Congress passed the Pure Food and Drug Act, which required manufacturers to disclose therapeutic ingredients on the drug label, and prohibited the sale of adulterated, misbranded, or deleterious products. The law presumed that, with accurate labeling, individuals could safely choose drugs. Advertising of therapeutic claims remained unregulated until the Shirley amendments in 1912 prohibited false and fraudulent statements regarding the curative or therapeutic effect of drugs."
Industry opposition blocked enactment of the Roosevelt administration’s 1933 bill to regulate drugs until a scandal in 1937. In order to improve the flavor of a sulfa-based drug called sulfanilamide, the Massengill Company added a chemical that was toxic, causing the rapid death of 106 people who had ingested the drug. Congress then passed the Food. Drug, and Cosmetic Act of 1938 [“FDCA”]. which required drug firms to seek FDA permission to market drugs, and which allowed the FDA 60 days to deny authorization if it found that the drug was dangerous or improperly labeled.
Manufacturers then had incentives to conduct research and to evaluate their products. The marketing of Thalidomide led to the birth of children with severe deformations in multiple countries, and created pressure for stronger regulation. The 1962 amendments to the FDCA prohibited marketing of drugs unless the FDA granted approval, and the amendments removed the 60 day deadline for FDA review of new drugs. The amendments required drug sponsors to demonstrate that drugs are effective—not only safe—for a designated use. It also authorizes the FDA to withdraw its approval for drugs already on the market based on new evidence. Manufacturers are required to track drug distribution to facilitate recalls of unsafe products, and to follow FDA standards for good manufacturing practices. The law restricts promotion of drugs to therapeutic uses approved by the FDA. Promotional materials must note risks, as well as benefits, and summarize side effects and contraindications. The FDA specifies what information the label must include, and labels must state the generic name, as well as the brand name.
In 1970, the FDA promulgated regulations that set standards for the evidence that manufacturers would have to submit in order to demonstrate that new drugs were safe and effective. Since then, testing of drugs follow set stages. After researchers have identified a potentially therapeutic molecule, they test its effects in laboratories on chemicals, cells, or tissues. The FDA then requires firms to test its drugs for toxicity on animals. Drug candidates that have not been ruled out due to toxicity or lack of efficacy can then be tested on humans in three phases. In Phase I. researchers test the drug on a small number of human subjects only to determine whether it is toxic in humans, and if so, at what doses. Phase II testing consists of a clinical trial in a larger group of patients in order to measure its benefits and risks. Drugs that are not highly toxic are tested in Phase III clinical trials on a large number of human research subjects, and researchers then compare its effect with a control group. Typically, the control group uses a placebo or an alternative therapy. Human subjects are randomly assigned to either the test group or the control group. It is a double blind study, which means that the medication must be coded so that neither the physician who administers the drug, nor the individual taking the drug, knows which individuals receive the test drug and which individuals receive the placebo [or the standard therapy to which it is compared] until the code is broken after collection of data. To counter the risk of fraud or unreliable studies, regulations establish standards for research methods, record keeping, and data reporting. The FDA also inspects toxicological laboratories and facilities that conduct clinical trials in order to monitor compliance.
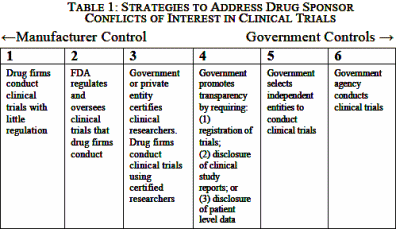
B. Options for Control of Clinical Trials
There are six options for addressing conflicts of interest in clinical trials, which are displayed in Table 1 below. At one extreme, the drug sponsor has complete control over clinical trials; at the other extreme, the federal government conducts the clinical trials. Between these two poles are four strategies that can be used individually or combined. The FDA relies mainly on the second strategy, which has been supplemented in recent years by the fourth strategy…
Drug manufacturers face a fundamental conflict of interest. Pursuit of profit compromises drug manufacturers" impartial assessment of the risks and benefit of their drugs. Their biased evaluation can corrupt public knowledge of drugs, lead to marketing unsafe and/or ineffective drugs, and undermine rational physician prescribing. Over the last century, federal regulation has mitigated, but not eliminated, this problem…
Right now, we’re operating in what Rodwin calls "the fourth strategy" – regulation. And it hasn’t gone so very well. At least in psychiatry, we’ve had 25 years of grossly exaggerated indications, efficacy reports, and safety claims. And even now that these medications’ failings are more publicly known, mental health care policy is still being formulated with these meds as the central strategy [eg collaborative care], metastacizing into the domain of primary care – a particularly shaky idea in my [and many other’s] opinion.
Fifth strategy?
Sounds like the government turning over the healthcare industry to managed care in order to get it out of the hands of greedy doctors. No conflict of interest there at all is there? Not enough conflict of interest – add PBMs.
If the author really has the premise that conflict of interest doesn’t go away, why would it disappear with “independent entities”. As far as I can tell many of these entities are far less accountable than Big Pharma.
And while I am at it – somebody needs to point out that practically all government regulation, laws, and statutes seem to benefit the legal profession. Maybe we should start with a law that all Statutes, new laws, and regulations be written jargon free – so you don’t need to be a lawyer to read them.
There are better models out there for industry regulation. As far as I can tell, if the proposed models do not currently exist – good luck with that. Maybe some day we will also be able to conduct massive clinical trials as suggested by Ioannidis.
It seems like there is an endless supply of impractical ideas. With a current federal debt of $19.3 trillion – the staffing at the existing FDA is probably at risk.
I totally agree with the above comment. The feds ruin almost everything they touch and EHR in ACA is proof. The FDA is very unimpressive and has its own agendas.
http://www.investors.com/politics/commentary/is-modern-science-polluted/
regulations don’t work, they only creative gaming incentives
what does work is tens of thousands of physicians unsubscribing to garbage journals
When this subject comes up, one of my first thoughts is to reflect back to the history of the 20th century in drug development. Say what you will about greedy pharmaceutical corporations and greedy KOLs who cut corners, the capitalist model delivered results – spectacular results – while the socialist model was a complete failure. I think it is safe to say that in its entire 80 year history the Soviet Union did not develop a single drug that the rest of the world had any use for.
In saying that I do not minimize the ethical issues of today in the industry. But we do sometimes overlook the large number of non-conflicted clinical scientists because the greedy KOLs grab so much attention. Here is a new discussion of one of the safety mechanisms in place for clinical trials. The members of these data monitoring committees get remarkably little credit.