by Gerardo Villarreal, Mark B. Hamner, José M. Cañive, Sophie Robert, Lawrence A. Calais, Valerie Durklaski, Yusheng Zhai, and Clifford QuallsAmerican Journal of Psychiatry. 2016 173[12]:1205-1212.
Received: July 27, 2015 Accepted: May 02, 2016 ClinicalTrials.gov Identifier: NCT00237393 Study Start Date: August 2003 Study Completion Date: December 2007
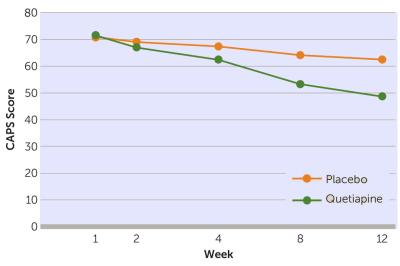
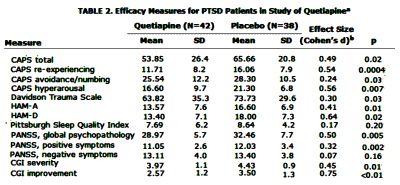
Objective: This was a 12-week randomized, placebo-controlled trial to assess the efficacy of quetiapine monotherapy in the treatment of posttraumatic stress disorder [PTSD].Method: Eighty patients were randomly assigned to treatment with either quetiapine or placebo. The primary outcome measure was the Clinician-Administered PTSD Scale [CAPS]. Secondary efficacy measures included the CAPS subscales, the Davidson Trauma Scale, the Positive and Negative Syndrome Scale [PANSS], the Clinical Global Impressions [CGI] scales for severity of Illness and improvement, the Hamilton Depression Rating Scale [HAM-D], and the Hamilton Anxiety Rating Scale [HAM-A]. Safety measurements included adverse events, vital signs, the Abnormal Involuntary Movement Scale, the Barnes Akathisia Scale, the Simpson-Angus Scale, and the Arizona Sexual Experiences Scale.Results: After a 1-week placebo run-in, quetiapine was started at a daily dosage of 25 mg and increased to a maximum of 800 mg; the average was 258 mg [range, 50–800 mg]. Reductions in CAPS total, re-experiencing, and hyperarousal scores were significantly greater for the quetiapine group than for the placebo group. Greater improvements were also observed for quetiapine in scores on the Davidson Trauma Scale, CGI severity and improvement ratings, PANSS positive symptom and general psychopathology subscales, HAM-A, and HAM-D than for placebo. Adverse events were generally mild and expected based on prior studies of quetiapine in this and other patient population. There were no differences in safety measures between groups.Conclusion: Quetiapine monotherapy was efficacious in the treatment of PTSD. These findings suggest quetiapine as a single agent is effective in treating military PTSD.
Presented at the 29th Annual Conference of the Anxiety Disorders Association of America, Santa Anna Pueblo, N.M., March 12–15, 2009, and the 2009 CINP Thematic Meeting, Edinburgh, April 25–27, 2009.
Funded by an investigator-initiated grant from AstraZeneca to Dr. Hamner.
Dr. Hamner has current research support from Alkermes and Pfizer; he has been the recipient of research grant support or honoraria and/or has served as a consultant for the following pharmaceutical companies: Abbott, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Forest Laboratories, Janssen, Lundbeck, Organon, Otsuka, and Sanofi-Synthlabo. Dr. Cañive has received research grant support or honoraria and/or has served as a consultant for the following pharmaceutical companies: Abbott, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Organon, Otsuka, and Sanofi-Synthlabo. The other authors report no financial relationships with commercial interests.
by Murray B. SteinAmerican Journal of Psychiatry. 2016 173[12]:1165-1166.
…the study by Villarreal et al., also in this issue, digs up a topic thought to be long dead: the efficacy of atypical antipsychotics for PTSD. Whereas some early, small trials had suggested that atypical antipsychotics, such as olanzapine or risperidone, might be useful adjunctive [to selective serotonin reuptake inhibitors] treatment for PTSD, a large VA Cooperative Study seemed to put the nail in the coffin of that treatment option by failing to show a benefit of adjunctive risperidone on global severity of PTSD. That trial, it should be noted, did show statistically significant [though, it was argued, clinically modest] beneficial effects on hyperarousal symptoms and, in a more recent secondary analysis, sleep disturbance. Moreover, meta-analysis suggests that atypical antipsychotics can be useful in treating PTSD, and clinicians continue to believe they are efficacious and continue to prescribe them for many [up to 20% of] patients with PTSD.Against this backdrop of high antipsychotic prescribing for PTSD in the face of limited evidence of efficacy comes this new randomized placebo-controlled trial of quetiapine as monotherapy for military-related PTSD [by Villarreal et al]. Well, not really a new study, but an old study — completed in 2008 — newly published. It is unclear why it took nearly a decade to publish the results of this positive study, but it is timely nonetheless. Albeit a fairly small trial that enrolled 80 patients, the results suggest that quetiapine can be useful in the treatment of PTSD, though the authors remind readers that adverse effects of this class of drugs must be considered in the risk-benefit ratio of whether to treat a given individual.
Whereas clinical practice should rarely be influenced by findings from a single trial, the results of this study should hasten a reinvigoration of research into the safety and efficacy of atypical antipsychotics for the treatment of PTSD. Though we yearn for a future where better and safer drugs for PTSD can be targeted for precisely the patients most likely to benefit from them with the fewest adverse effects, we are constrained to practice medicine in the present. Call it imprecision medicine if you must, but if atypical antipsychotics — administered as monotherapy or as adjunctive therapy — can help some patients with PTSD — then let’s shore up the evidence base for their utility so that we practitioners can feel less stressed about using them.
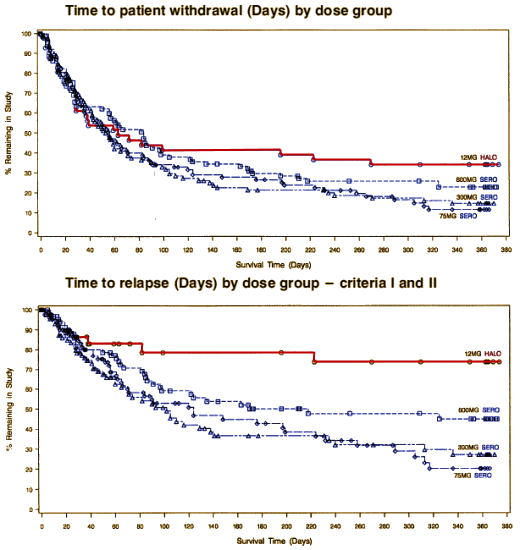
Washington PostBy Shankar VedantamMarch 18, 2009The study would come to be called "cursed," but it started out just as Study 15. It was a long-term trial of the antipsychotic drug Seroquel. The common wisdom in psychiatric circles was that newer drugs were far better than older drugs, but Study 15’s results suggested otherwise.
As a result, newly unearthed documents show, Study 15 suffered the same fate as many industry-sponsored trials that yield data drugmakers don’t like: It got buried. It took eight years before a taxpayer-funded study rediscovered what Study 15 had found — and raised serious concerns about an entire new class of expensive drugs. Study 15 was silenced in 1997, the same year Seroquel was approved by the Food and Drug Administration to treat schizophrenia…
We should compliment your tenacity in exposing what went on in so many trials. Pharma is by now so practiced in deceptive/misleading techniques of putting lipstick on their pigs that something like reverse engineering is the only way to get at the truth. And reverse engineering is a heck of a lot more onerous to pull off than is the original deception. Well done!
One thing that I always wonder about is whether severe PTSD could be easily confused as being certain subtypes of schizophrenia, I know anxiety is the feature that seemed to overwhelm the picture of my loved one, but when I asked about ‘PTSD (from a very experienced and ethical psychiatrist), she said for a PTSD diagnosis, the underlying cause has to be so extreme (war, abuse, etc.). I continue to wonder about that distinction, and found Noel Hunter’s discussion of this issue very interesting (She wrote a couple of blogs about it on MIA and talked about how uncomfortable the discussion made the psychologists who were presenting the trauma research.). This seems particularly significant to me in terms of when antipsychotics are used for ‘treatment resistant’ schizophrenia, and whether their use could be making the recovery more complicated. If any one could point me in the direction of useful research or articles or information about this topic – or had any thoughts to share about it , I would be very grateful
..just an addition to my above comment…The Noel Hunter blog is entitled something like “Trauma and Schizophrenia, the ultimate Political Battle (I wish the title was different!). The issue she speaks of is how can one differentiate between ‘dissociation’ and ‘psychosis’ in certain instances and goes on to give the following description of ‘dissociation’ which does sound to me like ‘psychosis’: “….how experiencing multiple traumas (unlike a single trauma) results in a shutting down response rather than hyperarousal and increased physiological activity. This includes: loss of emotion, loss of memory and language, shutting off of cognitive processing, deactivation of the brain, loss of physical sensation, social disengagement, miscommunication, and social withdrawal (McTeague, et al., 2010).”
Mickey,
Although I certainly agree with you that the upfront use of quetiapine for garden variety insomnia and anxiety has a poor risk benefit ratio there are people who benefit from it. In Minnesota any antipsychotic prescription requires written informed consent signed by both the physician and the patient. In going over hundreds of those consents in the past several years I have observed the following:
1. The weight gain/metabolic side effects are a nonstarter and immediately separates casual use from something else.
2. There are significant numbers of people the use quetiapine because nothing else works for insomnia, anxiety, bipolar disorder and even PTSD. All of the people I have seen in the past 6 years have had addictions and benzodiazepines and the z-drugs represent a bigger risk to them in terms of immediate life threatening consequences than quetiapine. In that context, many people prefer the medication and are willing to risk the significant side effects.
The biggest problem with PTSD treatment at this time is not the lack of antipsychotics but the lack of availability of exposure therapy and a step-wise approach that gets access to that therapy closer to the clinic intake.
I can’t figure out why anyone would try Seroquel before Prazosin for PTSD. A lot of vets have HTN and BPH as well so its a win-win-win. Granted it’s not that efficacious but meds for PTSD generally aren’t and it’s about as good as we have even tho off label. I could see using Seroquel after other options have failed.
As a forensic psychiatrist, I see TD lawsuits and I think avoiding antipsychotics unless necessary is the wisest choice.
Conceptually (disclaimer, this is a gross oversimplification) I see PTSD related more to the autonomic system than the dopamine system, and a completely distinct categorical entity from schizophrenia. Furthermore (oversimplification again), one is largely environmental and the other is largely environmental. Yes, I know there are different vulnerabilities, but the core of the disorder is trauma.
Seroquel for EVERYTHING is a huge problem at the VA. I hope the new VA secretary addresses whatever clinical subculture leads to these kind of decisions.
George and James,
Thanks for the comments. My military PTSD experience was post Viet Nam and I agree that in many of those cases, anxiety can be malignant. I expect that would have had me looking a the drugs you mention. The Z’s were certainly a big problem. In practice, I saw a lot more “domestic” trauma than I would’ve imagined. But that’s a different coindition that required a differing approach. All those Seroquel cases in the clinic were depression or anxiety symptom cases…
James,
I gather that Prazosin is one of those “worth a try” drugs…
I agree.
PTSD and schizophrenia are categorically very different and distinct, and I don’t see any theoretical benefit to using SGAs for PTSD. It’s not like the kind of obvious intuit using Bipolar meds for Bipolar II.
As a corollary I’m not a fan of using SGAs for nonpsychotic agitation either, unless of course nothing else works.
Prazosin protocol I use is from:
Am J Psychiatry 2013;170: 1003-1010.
Used for combat related PTSD in active duty soldiers. I have never come close to the max doses that the authors suggest for men and women in civilians. Also reminded me of past cardiology experience and the old theories of how to improve cardiac output by afterload reduction. In those days prazosin was typically that drug. After seeing how little it moves BP – I wonder how much afterload reduction was ever accomplished.
Wonder how much interest there would be in Prazosin if it weren’t off patent…
Probably there would be an effort to shoehorn it into every anxiety disorder…