I’ve been writing about Rapastinel, an NMDA receptor partial blocker touted to have Ketamine’s antidepressant effects without being a psychomimetic [see a touch of paralysis… and a block-buster-in-training…]. It was developed by a Northwestern University neuroscientist who formed a private company [Naurex], later purchased by industry giant Allergan for $560 M. They’ve recently registered four phase 3 clinical trials – all now recruiting. Before discussing these trials, here’s a bit of a review.
|
A properly conducted clinical trial has a number of essential elements related to the efficacy analysis:
Many clinical trials have been misreported in journal articles, and a common modus operandi for distorting the results has been to base the report on the outcome variable that give the most favorable results rather than those declared a priori. Since the pharmaceutical companies that sponsor these trials insist that the raw data is proprietary [a secret], we don’t have the IPD [Individual Participant Data], the CRFs [Clinical Report Forms], the Protocol, or the SAP [Statistical Analysis Plan]. So our only shot at knowing what outcome variables were designated primary and secondary a priori is the registration on ClinicalTrials.gov. And even registration is only useful if it’s before the study starts and the outcome variables are clearly stated.
|
And so to the Rapastinel Phase 3 trials. Three of the four are virtual clones of each other, differing only in the number of subjects [why?] and one has a second dose level. They all have the same cohort – subjects in a Major Depressive Episode that "[h]ave no more than partial response [< 50% improvement] to ongoing treatment with a protocol-allowed antidepressant." These three trials call for 4589 total subjects who have failed to respond to SSRIs, documented by a less that 50% response on some unspecified rating scale after a course of an antidepressant from some unspecified list of drugs. That’s a huge cohort and it’s unclear why so many. There’s no power analysis included to explain it. Likewise, there’s a huge number of sites [134]. I even wondered if they are piggy-backing on the end of a bunch of somebody else’s antidepressant trials to get cases? Some kind of explanation would be welcome.
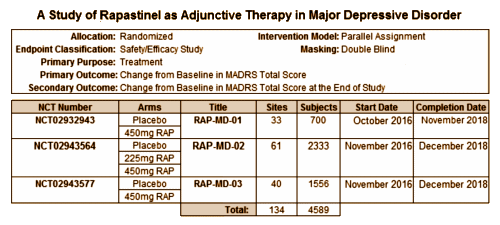
This is a medication that is given intravenously and the antidepressant effect lasts ~one week in the proof-of-concept study [see a block-buster-in-training…], so they call for weekly injections. Here’s all they have to say about the conduct of the study and the primary and secondary outcome variables:
-
Primary Outcome Measure: Change from Baseline in Montgomery-Asberg Depression Rating Scale [MADRS] Total Score [Time Frame: Baseline and 1 Day]
-
Secondary Outcome Measure: Change from Baseline in MADRS Total Score at the End of Study [Time Frame: Baseline and 3 Weeks]
How long does the study last? It doesn’t really say. I’m presuming three weeks based on that Time Frame comment in the Secondary Outcome Measure. They say the Primary Outcome Measure is the MADRS Score on day 1. After the first injection? or after each injection? And why day 1? After studying it a while, this was my best guess about what they were going to do,,,
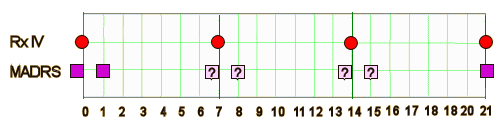
Looking at the only published study [see a block-buster-in-training…], it seems important to take a look later in that week. That’s the whole reason the drug’s being given – for its presumed ability to last the week. And there are any number of Baselines. Which one is used? When? Is there a minimum MADRS score for entering the study? I could go on and on [and so could you].
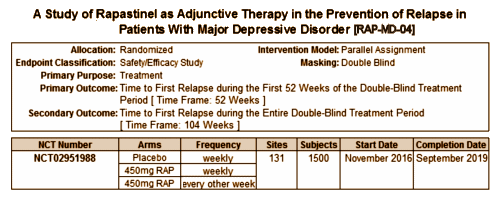
And the 4th trial? RAP-MD-04? While it doesn’t say it in the ClinicalTrials.gov registration document, this trial apparently follows some subset of the subjects from the other three trials [responders?] on either continued Placebo or Rapastinel [weekly or every two weeks] as a relapse prevention trial for two years. And though it presumably follows some group from RAP-MD-01, RAP-MD-02, and RAP-MD-03, the criteria for that determination isn’t specified. Again, there’s no self rating depression scale, no PANSS, no adverse event plan mentioned.
My reaction was that both Allergan and ClinicalTrials.gov deserve frowns for this. The Allergan material was frankly incompetent, and the Federal agency should have refused to accept such a poor quality posting by Allergan.