F.D.A. Approval of Seroquel
The information on this page comes from the text [selected quotes] and tables [expressed graphically] in the FDA Statistical Review and Evaluation of Seroquel [Quietipine] dated Februrary 7, 1997. Statistically significant values are circled in green.
Introduction
"The sponsor has submitted three (3) six-week adequate, randomized, placebo-controlled trials in support of Seroquel’s efficacy and safety in patients with acte exacerbations of schizophrena. A fourth trial, 0012 compared 450mg tid, 450mg bid, and 50mg bid. Trial 0001/0008 enrolled patients in the US and Europe. Trial 0006 enrolled patients in the US, Trial 0013 enrolled patients in the US and Canada, and Trial 0012 enrolled patients from Europe, Israel, and Canada."
Trial 0006
"This Phase II trial consisted of one flexible dosing arm of Seroquel (75 mg/day – 750 mg/day) and a placebo arm. The protocol called for 50 patients per arm in order to have 90% power to detect an 8 point difference in change from baseline BPRS between the Seroquel arm and placebo. A total of 109 patients were randomized (Seroquel N=54, Placebo N=55) among 11 centers in the US. However, 3 patients had no post-baseline data leaving 53/group available for analysis. For the 106 patients analyzed, the completion rates were 53% for Seroquel and 42% for placebo."
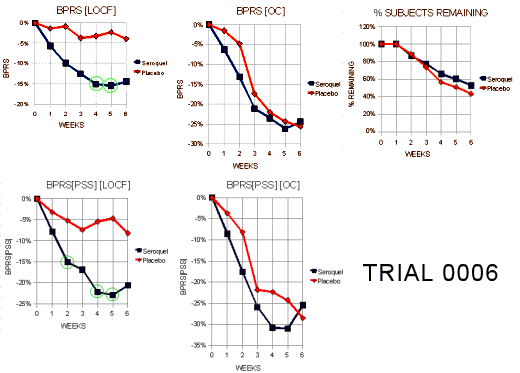
"Tables l0a and l0b display the visit-wise results of the total BPRS for the LOCF and observed cases analyses, respectively. The borderline statistical result for the LOCF is likely due to the less than anticipated treatment difference at 6 weeks (-8.1 vs -2.1). Note that there was no difference at all for the completers. Thus, Figure 5 indicates that the entire treatment difference in the LOCF analysis is due to dropouts in the first 4 weeks of the trial."
Trial 0008
"This 6-week study randomized a total of 286 patients among 37 centers in the US (59%) and Europe (41 %): N=96 High dose, N=94 Low dose, N=96 Placebo. This is more than 50% greater then the number ‘evaluable’ patients called for in the protocol… A total of 4 patients had no post-baseline data, so that 282 patients comprise the effcacy ITT population. The endpoints of interest are BPRS total, key BPRS, CGI Severity and the negative PANSS (only in Europe) and the SANS (done only in the US). The protocol states that ANCOVA with baseline as the covariate would be used for all analyses. However, there is no plan for multiple comparisons among treatment arms. Table 1 displays the baseline conditions and demographics of the treatment groups. Completion rates were 50% for High dose, 43% for Low dose, and 41% for Placebo. ‘Treatment failure’ accounted for between 40%-50% of the dropouts."
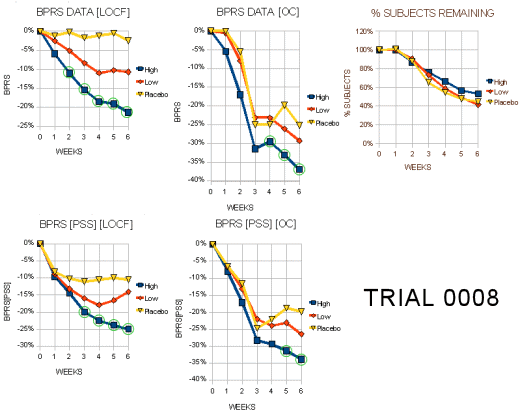
"Tables 3a and 3b display the visit-wise results of the total BPRS for the LOCF and observed cases analyses, respectively. It is not surprising that the high dose is statistically significant but the low dose is not, since the trial was planned (power= 90%) to find a 9 point difference between the high dose and placebo arms for the BPRS… Tables 5a and 5b display the results of the key positive symptom BPRS cluster for the LOCF and observed cases, respectively."
Trial 0012
"The intent of this trial was to show whether either dosing regimen of 450mg/day (tid vs bid) was better than the low dose (50mg/day), and whether the the higher dose regimens differed from each other. The trial was planned so that 170 patients/arm would provide 90% power to detect, a 6 point difference in change from baseline of the total BPRS between any two pairs of treatment arms. A total of 618 patients were randomized with 596 contributing at least one post-baseline observation on the BPRS… Approximately 50% of all patients completed the 6 week triaL. There was no relationship between dose and frequency of dropout due to lack of effcacy…"
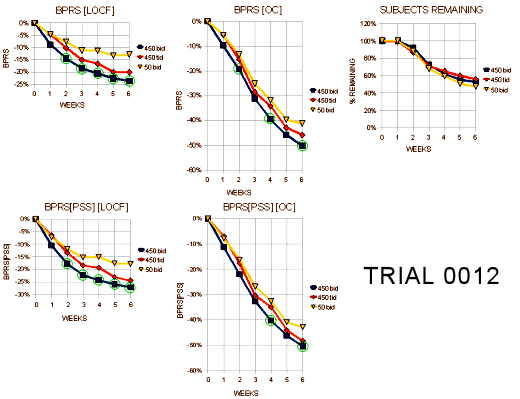
"Tables 22a and 22b display the visit-wise results of the total BPRS for the LOCF and observed cases analyses, respectively. There was no plan for multiple comparisons in the protocol. However, the p=.005 for the bid dosage is suffcient for statistical significance. Also, the p=.05 for the tid dosage would be just significant using Fisher’s LSD. The confidence interval for the difference between the bid and tid dosages at 450mg rule out a 5-6 point average difference for the total BPRS. Tables 24a and 24b display the results of the key positve symptom BPRS cluster for the LOCF and observed cases, respectively. The bid regimen is significant for both the LOCF and observed cases analyses."
Trial 0013
"This Phase III trial randomized a total of 361 patients among 26 in the US and Canada to 5 fixed doses of Seroquel (75 mg N=53, 150 mg N=48, 300 mg N=52, 600 mg N=51, 750 mg N=54) or 12 mg haloperidol (N=52) or placebo (N=51). The target sample size of 50/arm resulted from simulations of various dose-response models using a power of90%."
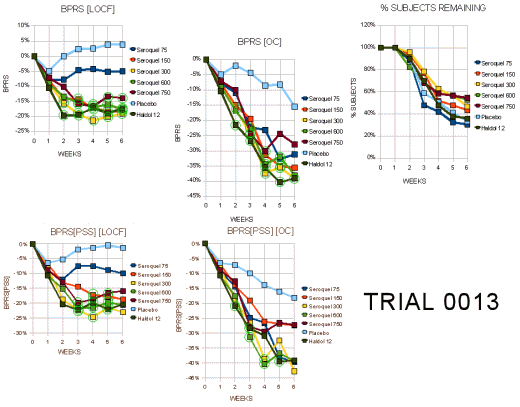
"Withdrawal rates ranged from 50%-70% with the highest in the placebo, 75 mgg and haloperidol groups. Tables 16a and 16b display the visit-wise results of the total BPRS for the LOCF and observed cases analyses, respectively. All but the 75 mg/day dose of Seroquel were statistically significantly different from placebo for the LOCF analysis, whereas none were significant in the completers analysis… Tables 18a and 18b display the results of the key positive symptom BPRS cluster for the LOCF and observed cases, respectively. This endpoint shows a similar profile of a nearly significant LOCF analysis and a null completers analysis."
Discussion
"Trials 0008 and 0013 clearly demonstrate statistically signicantly differences between Seroquel and placebo. Both trials were able to demonstrate treatment differences of at least 4 total BPRS points in completers at six weeks. The reasons for the null results for completers in trial 0006 are unclear. The median doses of the Seroquel completers at the end of the trials were simar in both trials 0006 and 0008, another dose-escalation trial (450 rng and 500mg, respectively). The major difference was in the pedormance ofthe placebo groups. The placebo group in trial 0006 decreased from baseline an average of 13.9 points while the placebo average in trial 0008 was 9.7, making it more difficult to show a difference in trial 0006. As a checkon trial 0008, this reviewer analysed ony the first 165 randomized patients and foundthe treatment difference to be comparable to that using all patients. In a submission dated September 24, 1996, the sponsor reanalyzed the data without Dr. Borison’s center. Omitting Dr. Borison’s center had no substantial effect on the results."